Sponge: Difference between revisions
→Fossil record: Fixed grammar Tags: Mobile edit Mobile app edit Android app edit |
ZKevinTheCat (talk | contribs) No edit summary |
||
| (87 intermediate revisions by 45 users not shown) | |||
| Line 4: | Line 4: | ||
{{Good article}} |
{{Good article}} |
||
{{Automatic taxobox |
{{Automatic taxobox |
||
| name |
| name=Sponges |
||
| taxon |
| taxon=Porifera |
||
| fossil_range=[[Ediacaran]]-present;<ref>{{cite journal | last1 = Brasier | first1 = Martin | last2 = Green | first2 = Owen | last3 = Shields | first3 = Graham | date = April 1997 | title = Ediacarian sponge spicule clusters from southwestern Mongolia and the origins of the Cambrian fauna |url=https://www.researchgate.net/publication/240669632 |format=PDF | journal = Geology | volume = 25 | issue = 4 | pages = 303–306 | doi = 10.1130/0091-7613(1997)025<0303:ESSCFS>2.3.CO;2 | bibcode = 1997Geo....25..303B }}</ref> probable [[Cryogenian]] record,<ref>{{cite journal |last1=Gold |first1=David |last2=Grabenstatter |first2=Jonathan |last3=de Mendoza |first3=Alex |last4=Riesgo |first4=Ana |last5=Ruiz-Trillo |first5=Iñaki |last6=Summons |first6=Roger |title=Sterol and genomic analyses validate the sponge biomarker hypothesis |journal=PNAS |date=22 February 2016 |doi=10.1073/pnas.1512614113 |url=https://www.pnas.org/doi/full/10.1073/pnas.1512614113}}</ref> {{Long fossil range |earliest=650|544|0}} |
|||
| fossil_range = {{fossil range|Ediacaran|recent}} |
|||
| image |
| image=Aplysina archeri (Stove-pipe Sponge-pink variation).jpg |
||
| image_caption |
| image_caption=A [[Aplysina archeri|stove-pipe sponge]] |
||
| authority |
| authority=[[Robert Edmund Grant|Grant]], 1836 |
||
| subdivision_ranks |
| subdivision_ranks=Classes |
||
| subdivision |
| subdivision=* [[Calcarea]] |
||
* [[Demospongiae]] |
* [[Demospongiae]] |
||
* [[Hexactinellida]] |
* [[Hexactinellida]] |
||
| Line 18: | Line 18: | ||
* †"[[Heteractinida]]" <small>([[paraphyletic]])</small> |
* †"[[Heteractinida]]" <small>([[paraphyletic]])</small> |
||
* †[[Stromatoporoidea]] |
* †[[Stromatoporoidea]] |
||
| synonyms |
| synonyms=[[Parazoa]]/Ahistozoa (''sans'' [[Placozoa]])<ref>{{Cite journal|journal=Ethnolinguistic|last=Pajdzińska |first=A. |title=Animals die more shallowly: they aren't deceased, they're dead. Animals in the polish linguistic worldview and in contemporary life sciences |doi=10.17951/et.2017.29.135 |date=2018 |volume=29 |pages=147–161 |doi-access=free}}</ref> |
||
}} |
}} |
||
'''Sponges''' or '''sea sponges''' are members of the [[metazoan]] [[phylum]] '''Porifera'''<ref>{{cite web |url=https://www.marinespecies.org/aphia.php?p=taxdetails&id=558 |title=Porifera |date=2024 |website= World Register of Marine Species |publisher=Flanders Marine Institute |access-date=8 May 2024}}</ref> ({{IPAc-en|p|ə|ˈ|r|ɪ|f|ər|ə|,|_|p|ɔː|-}} {{respell|pər|IF|ər|ə|,_|por|-}}; meaning 'pore bearer'),<ref>{{Cite Merriam-Webster|porifera|accessdate=2024-05-12}}</ref> a [[basal animal]] [[clade]] and a [[sister taxon]] of the [[Eumetazoa|diploblasts]].<ref name="Feuda_2017">{{cite journal |last1=Feuda|first1=Roberto|last2=Dohrmann|first2=Martin|last3=Pett|first3=Walker|last4=Philippe|first4=Hervé|last5=Rota-Stabelli|first5=Omar|last6=Lartillot|first6=Nicolas|last7=Wörheide|first7=Gert|last8=Pisani|first8=Davide |title=Improved Modeling of Compositional Heterogeneity Supports Sponges as Sister to All Other Animals |journal=Current Biology |volume=27 |issue=24 |pages=3864–3870.e4 |date=December 2017 |pmid=29199080 |doi=10.1016/j.cub.2017.11.008 |doi-access=free |bibcode=2017CBio...27E3864F }}</ref> They are [[sessility (motility)|sessile]] [[filter feeder]]s that are bound to the [[seabed]], and are one of the most ancient members of [[macrobenthos]], with many historical species being important [[sponge reef|reef]]-building organisms. |
|||
'''Sponges''', the members of the [[phylum]] '''Porifera''' ({{IPAc-en|p|ə|ˈ|r|ɪ|f|ər|ə}}; meaning 'pore bearer'), are a [[basal animal]] [[clade]] as a sister of the [[Eumetazoa|diploblasts]].<ref name="Feuda_2017">{{cite journal | vauthors = Feuda R, Dohrmann M, Pett W, Philippe H, Rota-Stabelli O, Lartillot N, Wörheide G, Pisani D | display-authors = 6 | title = Improved Modeling of Compositional Heterogeneity Supports Sponges as Sister to All Other Animals | journal = Current Biology | volume = 27 | issue = 24 | pages = 3864–3870.e4 | date = December 2017 | pmid = 29199080 | doi = 10.1016/j.cub.2017.11.008 | doi-access = free }}</ref><ref>{{cite journal | vauthors = Pisani D, Pett W, Dohrmann M, Feuda R, Rota-Stabelli O, Philippe H, Lartillot N, Wörheide G | display-authors = 6 | title = Genomic data do not support comb jellies as the sister group to all other animals | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 112 | issue = 50 | pages = 15402–7 | date = December 2015 | pmid = 26621703 | pmc = 4687580 | doi = 10.1073/pnas.1518127112 | bibcode = 2015PNAS..11215402P | doi-access = free }}</ref><ref name="Simion_2017">{{cite journal | vauthors = Simion P, Philippe H, Baurain D, Jager M, Richter DJ, Di Franco A, Roure B, Satoh N, Quéinnec É, Ereskovsky A, Lapébie P, Corre E, Delsuc F, King N, Wörheide G, Manuel M | display-authors = 6 | title = A Large and Consistent Phylogenomic Dataset Supports Sponges as the Sister Group to All Other Animals | language = en | journal = Current Biology | volume = 27 | issue = 7 | pages = 958–967 | date = April 2017 | pmid = 28318975 | doi = 10.1016/j.cub.2017.02.031 | url = https://hal.archives-ouvertes.fr/hal-01681528/file/Simion_etal2017_CurrBiol_proofs.pdf | type = <!-- Submitted manuscript --> | doi-access = free }}</ref><ref>{{Cite journal|last=Giribet|first=Gonzalo | name-list-style = vanc |date=1 October 2016|title=Genomics and the animal tree of life: conflicts and future prospects|journal=Zoologica Scripta |volume=45|pages=14–21|doi=10.1111/zsc.12215|issn=1463-6409|doi-access=free}}</ref><ref>{{cite bioRxiv| vauthors = Laumer CE, Gruber-Vodicka H, Hadfield MG, Pearse VB, Riesgo A, Marioni JC, Giribet G |date=2017-10-11|title=Placozoans are eumetazoans related to Cnidaria|biorxiv=10.1101/200972}}</ref> They are [[Multicellular organism|multicellular]] organisms that have bodies full of pores and channels allowing water to circulate through them, consisting of jelly-like [[mesohyl]] sandwiched between two thin layers of [[Cell (biology)|cells]]. |
|||
Sponges have unspecialized cells that can [[cellular differentiation|transform into other types]] and that often migrate between the main cell layers and the mesohyl in the process. |
Sponges are [[multicellular organism]]s consisting of jelly-like [[mesohyl]] sandwiched between two thin layers of [[cell (biology)|cells]], and usually have tube-like bodies full of pores and channels that allow water to circulate through them. They have unspecialized cells that can [[cellular differentiation|transform into other types]] and that often migrate between the main cell layers and the mesohyl in the process. They do not have complex [[nervous system|nervous]],<ref name="Moroz_2022">{{cite journal |last1=Moroz |first1=L.L. |last2=Romanova |first2=D.Y. |title=Alternative neural systems: What is a neuron? (Ctenophores, sponges and placozoans) |journal=Frontiers in Cell and Developmental Biology |volume=10 |pages=1071961 |date=23 December 2022 |pmid=36619868 |pmc=9816575 |doi=10.3389/fcell.2022.1071961 |doi-access=free }}</ref> [[digestive system|digestive]] or [[circulatory system]]s. Instead, most rely on maintaining a constant water flow through their bodies to obtain food and oxygen and to remove wastes, usually via [[flagella]] movements of the so-called "[[collar cell]]s". |
||
Believed to be some of the most [[basal (phylogenetics)|basal]] animals alive today, sponges were possibly the first [[outgroup (cladistics)|outgroup]] to branch off the [[evolutionary tree]] from the [[Urmetazoan|last common ancestor of all animals]],<ref name="Feuda_2017"/> with fossil evidence of primitive sponges such as ''[[Otavia]]'' from as early as the [[Tonian]] [[geologic period|period]] (around 800 [[million years ago|Mya]]). The branch of [[zoology]] that studies sponges is known as '''spongiology'''.<ref>{{cite Merriam-Webster|Spongiology|access-date=27 December 2017}}</ref>{{toclimit|3}} |
|||
== Etymology == |
== Etymology == |
||
The term ''sponge'' derives from the [[Ancient Greek]] word {{wikt-lang|grc|σπόγγος}} |
The term ''sponge'' derives from the [[Ancient Greek]] word {{wikt-lang|grc|σπόγγος}} {{grc-transl|σπόγγος}}.<ref>{{Cite dictionary |title=σπόγγος |url=https://www.perseus.tufts.edu/hopper/text?doc=Perseus:text:1999.04.0057:alphabetic+letter=*s111:entry+group=61:entry=spo/ggos |last1=Liddell |first1=Henry George |last2=Scott |first2=Robert |dictionary=A Greek-English Lexicon |year=1940 |via=Perseus |access-date=5 September 2021 |archive-url=https://web.archive.org/web/20210905062852/https://www.perseus.tufts.edu/hopper/text?doc=Perseus:text:1999.04.0057:alphabetic+letter=*s111:entry+group=61:entry=spo/ggos |archive-date=5 September 2021 |url-status=live}}</ref> The [[scientific name]] ''Porifera'' is a [[neuter gender|neuter]] [[plural]] of the [[Modern Latin]] term ''porifer'', which comes from the [[root (linguistics)|root]]s ''porus'' meaning "pore, opening", and ''-fer'' meaning "bearing or carrying". |
||
== Overview == |
== Overview == |
||
[[File:Reef3859 - Flickr - NOAA Photo Library.jpg|thumb|right|Sponge [[biodiversity]] and [[morphotype]]s at the lip of a wall site in {{convert|60|ft|m|-1}} of water. Included are the yellow tube sponge, ''[[Aplysina fistularis]]'', the purple vase sponge, ''[[Niphates digitalis]]'', the red encrusting sponge, ''[[Spirastrella coccinea]]'', and the gray rope sponge, ''[[Callyspongia]]'' sp.]] |
[[File:Reef3859 - Flickr - NOAA Photo Library.jpg|thumb|right|Sponge [[biodiversity]] and [[morphotype]]s at the lip of a wall site in {{convert|60|ft|m|-1}} of water. Included are the yellow tube sponge, ''[[Aplysina fistularis]]'', the purple vase sponge, ''[[Niphates digitalis]]'', the red encrusting sponge, ''[[Spirastrella coccinea]]'', and the gray rope sponge, ''[[Callyspongia]]'' sp.]] |
||
Sponges are similar to other animals in that they are [[Multicellular organism|multicellular]], [[heterotroph]]ic, lack [[cell wall]]s and produce [[sperm cell]]s. Unlike other animals, they lack true [[Tissue (biology)|tissues]]<ref name="Hooper">{{cite web |url= https://www.qm.qld.gov.au/microsites/biodiscovery/03sponges-and-corals/structure-of-sponges.html |title=Structure of Sponges |last= Hooper |first=J. |year=2018 |website=Queensland Museum |access-date=27 September 2019 |archive-url=https://web.archive.org/web/20190926205031/https://www.qm.qld.gov.au/microsites/biodiscovery/03sponges-and-corals/structure-of-sponges.html |archive-date=26 September 2019 |url-status=dead }}</ref> and [[Organ (anatomy)|organs]].<ref>{{cite journal |last1=Thacker |first1=Robert W. |last2=Díaz |first2=Maria Cristina |last3=Kerner |first3=Adeline |last4=Vignes-Lebbe |first4=Régine |last5=Segerdell |first5=Erik |last6=Haendel |first6=Melissa A. |last7=Mungall |first7=Christopher J. |title=The Porifera Ontology (PORO): enhancing sponge systematics with an anatomy ontology |journal=Journal of Biomedical Semantics |volume=5 |issue=1 |pages=39 |date=8 September 2014 |pmid=25276334 |pmc=4177528 |doi=10.1186/2041-1480-5-39 |doi-access=free }}</ref> Some of them are radially symmetrical, but most are asymmetrical. The shapes of their bodies are adapted for maximal efficiency of water flow through the central cavity, where the water deposits nutrients and then leaves through a hole called the [[osculum]]. The [[unicellular|single-celled]] [[choanoflagellate]]s resemble the [[choanocyte]] cells of sponges which are used to drive their water flow systems and capture most of their food. This along with phylogenetic studies of ribosomal molecules have been used as morphological evidence to suggest sponges are the sister group to the rest of animals.<ref name="Collins_1998">{{cite journal |last=Collins |first=A.G. |title=Evaluating multiple alternative hypotheses for the origin of Bilateria: an analysis of 18S rRNA molecular evidence |journal=Proceedings of the National Academy of Sciences of the United States of America |volume=95 |issue=26 |pages=15458–63 |date=December 1998 |pmid=9860990 |pmc=28064 |doi=10.1073/pnas.95.26.15458 |bibcode=1998PNAS...9515458C |doi-access=free }}</ref> A great majority are marine (salt-water) species, ranging in habitat from tidal zones to depths exceeding {{convert|8800|m|mi|abbr=on}}, though there are freshwater species. All adult sponges are [[Sessility (motility)|sessile]], meaning that they attach to an underwater surface and remain fixed in place (i.e., do not travel). While in their [[larvae|larval stage]] of life, they are [[motility|motile]]. |
|||
Sponges are similar to other animals in that they are [[Multicellular organism|multicellular]], [[heterotroph]]ic, lack [[cell wall]]s and produce [[sperm cell]]s. Unlike other animals, they lack true [[Tissue (biology)|tissues]]<ref name="Hooper">{{cite web |
|||
|url=https://www.qm.qld.gov.au/microsites/biodiscovery/03sponges-and-corals/structure-of-sponges.html |
|||
|title=Structure of Sponges |
|||
|last=Hooper |
|||
|first=John |
|||
|year=2018 |
|||
|website=Queensland Museum |
|||
|access-date=27 September 2019 |
|||
|archive-url=https://web.archive.org/web/20190926205031/https://www.qm.qld.gov.au/microsites/biodiscovery/03sponges-and-corals/structure-of-sponges.html |
|||
|archive-date=26 September 2019 |
|||
|url-status=dead |
|||
}}</ref> and [[Organ (anatomy)|organs]].<ref>{{cite journal |
|||
| last1=Thacker |
|||
| first1=Robert W |
|||
| last2=Diaz |
|||
| first2=Maria Christina |
|||
| date=8 September 2014 |
|||
| title= The Porifera Ontology (PORO): enhancing sponge systematics with an anatomy ontology |
|||
| journal=J Biomed Semantics |
|||
| volume=5 |
|||
| issue=39 |
|||
| pages=39 |
|||
| doi=10.1186/2041-1480-5-39 |
|||
| pmc=4177528 |
|||
| pmid=25276334 |
|||
| doi-access=free |
|||
}}</ref> Some of them are radially symmetrical, but most are asymmetrical. The shapes of their bodies are adapted for maximal efficiency of water flow through the central cavity, where the water deposits nutrients and then leaves through a hole called the [[osculum]]. Many sponges have internal skeletons of spicules (skeletal-like fragments of [[calcium carbonate]] or [[silicon dioxide]]), and/or [[spongin]] (a modified type of collagen protein).<ref name="Hooper" /> All adult sponges are [[Sessility (motility)|sessile]] aquatic animals, meaning that they attach to an underwater surface and remain fixed in place (i.e., do not travel) while in [[larvae|larval stage]] of life they are [[motility|motile]]. Although there are freshwater species, the great majority are marine (salt-water) species, ranging in habitat from tidal zones to depths exceeding {{convert|8800|m|mi|abbr=on}}. |
|||
Many sponges have internal skeletons of spicules (skeletal-like fragments of [[calcium carbonate]] or [[silicon dioxide]]), and/or [[spongin]] (a modified type of collagen protein).<ref name="Hooper"/> An internal gelatinous [[matrix (biology)|matrix]] called mesohyl functions as an [[endoskeleton]], and it is the only skeleton in soft sponges that encrust such hard surfaces as rocks. More commonly, the mesohyl is stiffened by [[Biomineralization|mineral]] [[sponge spicule|spicules]], by spongin fibers, or both. 90% of all known sponge species that have the widest range of habitats including all freshwater ones are [[demosponge]]s that use spongin; many species have [[silica]] spicules, whereas some species have calcium carbonate [[exoskeleton]]s. [[Calcareous sponge]]s have calcium carbonate spicules and, in some species, calcium carbonate exoskeletons, are restricted to relatively shallow marine waters where production of calcium carbonate is easiest.<ref name="Bergquist_1978"/>{{rp|179}} The fragile [[glass sponge]]s, with "[[scaffolding]]" of silica spicules, are restricted to polar regions and the ocean depths where predators are rare. Fossils of all of these types have been found in rocks dated from {{ma|580}}. In addition [[Archaeocyathid]]s, whose fossils are common in rocks from {{ma|530|490}}, are now regarded as a type of sponge. |
|||
Although most of the approximately 5,000–10,000 known species of sponges feed on [[bacteria]] and other microscopic food in the water, some host [[photosynthesis|photosynthesizing]] microorganisms as [[endosymbiont]]s, and these alliances often produce more food and oxygen than they consume. A few species of sponges that live in food-poor environments have evolved as [[carnivore]]s that prey mainly on small [[crustacean]]s.{{sfn|Vacelet|Duport|2004|pp=179–190}} |
|||
Although most of the approximately 5,000–10,000 known species of sponges feed on [[bacteria]] and other microscopic food in the water, some host [[photosynthesis|photosynthesizing]] microorganisms as [[endosymbiont]]s, and these alliances often produce more food and oxygen than they consume. A few species of sponges that live in food-poor environments have evolved as [[carnivore]]s that prey mainly on small [[crustacean]]s.<ref>{{cite journal |last1=Vacelet |first1=J. |last2=Duport |first2=E. |title=Prey capture and digestion in the carnivorous sponge ''Asbestopluma hypogea'' (Porifera: Demospongiae)|year=2004|journal=[[Zoomorphology]]|volume=123|issue=4|pages=179–190|s2cid=24484610 |doi=10.1007/s00435-004-0100-0 }}</ref> |
|||
Sponges reproduce both asexually and sexually. Most species that use [[sexual reproduction]] release [[sperm]] cells into the water to fertilize [[ovum|ova]] that in some species are released and in others are retained by the "mother". The fertilized eggs develop into [[larva]]e, which swim off in search of places to settle.{{sfn|Bergquist|1978|pp=183–185}} Sponges are known for regenerating from fragments that are broken off, although this only works if the fragments include the right types of cells. Some species reproduce by budding. When environmental conditions become less hospitable to the sponges, for example as temperatures drop, many freshwater species and a few marine ones produce [[gemmule]]s, "survival pods" of unspecialized cells that remain dormant until conditions improve; they then either form completely new sponges or recolonize the skeletons of their parents.{{sfn|Bergquist|1978|pp=120–127}} |
|||
Most sponges reproduce [[sexual reproduction|sexually]], but they can also reproduce asexually. Sexually reproducing species release [[sperm]] cells into the water to fertilize [[ovum|ova]] released or retained by its mate or "mother"; the fertilized eggs develop into [[larva]]e which swim off in search of places to settle.<ref name="Bergquist_1978">{{Cite book |last=Bergquist |first=P. R. |year=1978 |title=Sponges |publisher=Hutchinson |location=London |isbn=978-0-520-03658-1}}</ref>{{rp|183–185}} Sponges are known for regenerating from fragments that are broken off, although this only works if the fragments include the right types of cells. Some species reproduce by budding. When environmental conditions become less hospitable to the sponges, for example as temperatures drop, many freshwater species and a few marine ones produce [[gemmule]]s, "survival pods" of unspecialized cells that remain dormant until conditions improve; they then either form completely new sponges or recolonize the skeletons of their parents.<ref name="Bergquist_1978"/>{{rp|120–127}} |
|||
In most sponges, an internal gelatinous matrix called mesohyl functions as an [[endoskeleton]], and it is the only skeleton in soft sponges that encrust such hard surfaces as rocks. More commonly, the mesohyl is stiffened by [[Biomineralization|mineral]] [[sponge spicule|spicules]], by spongin fibers, or both. [[Demosponge]]s use spongin; many species have [[silica]] spicules, whereas some species have calcium carbonate [[exoskeleton]]s. [[Demosponges]] constitute about 90% of all known sponge species, including all freshwater ones, and they have the widest range of habitats. [[Calcareous sponge]]s, which have calcium carbonate spicules and, in some species, calcium carbonate exoskeletons, are restricted to relatively shallow marine waters where production of calcium carbonate is easiest.{{sfn|Bergquist|1978|p=179}} The fragile [[glass sponge]]s, with "[[scaffolding]]" of silica spicules, are restricted to polar regions and the ocean depths where predators are rare. Fossils of all of these types have been found in rocks dated from {{ma|580}}. In addition [[Archaeocyathid]]s, whose fossils are common in rocks from {{ma|530|490}}, are now regarded as a type of sponge. |
|||
[[File:Choanoflagellate and choanocyte.png|thumb|upright=1.8| |
[[File:Choanoflagellate and choanocyte.png|thumb|upright=1.8|Cells of the protist choanoflagellate clade closely resemble sponge choanocyte cells. Beating of choanocyte [[flagella]] draws water through the sponge so that nutrients can be extracted and waste removed.<ref>Clark, M.A.; Choi, J.; Douglas, M. (2018) [https://d3bxy9euw4e147.cloudfront.net/oscms-prodcms/media/documents/Biology2e-OP.pdf ''Biology 2e'']{{dead link|date=July 2020 |bot=InternetArchiveBot |fix-attempted=yes }}, page 776, ''OpenStax''. {{ISBN|978-1-947172-52-4}}.</ref>]] |
||
The few species of demosponge that have entirely soft fibrous skeletons with no hard elements have been used by humans over thousands of years for several purposes, including as padding and as cleaning tools. By the 1950s, though, these had been [[overfishing|overfished]] so heavily that the industry almost collapsed, and most sponge-like materials are now synthetic. Sponges and their microscopic endosymbionts are now being researched as possible sources of medicines for treating a wide range of diseases. [[Dolphin]]s have been observed using sponges as tools while [[foraging]].<ref name="Krutzen_2005"/> |
|||
The [[unicellular|single-celled]] [[choanoflagellate]]s resemble the choanocyte cells of sponges which are used to drive their water flow systems and capture most of their food. This along with phylogenetic studies of ribosomal molecules have been used as morphological evidence to suggest sponges are the sister group to the rest of animals.<ref name="collins 1998">{{cite journal | vauthors = Collins AG | title = Evaluating multiple alternative hypotheses for the origin of Bilateria: an analysis of 18S rRNA molecular evidence | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 95 | issue = 26 | pages = 15458–63 | date = December 1998 | pmid = 9860990 | pmc = 28064 | doi = 10.1073/pnas.95.26.15458 | bibcode = 1998PNAS...9515458C | doi-access = free }}</ref> |
|||
The few species of demosponge that have entirely soft fibrous skeletons with no hard elements have been used by humans over thousands of years for several purposes, including as padding and as cleaning tools. By the 1950s, though, these had been [[overfishing|overfished]] so heavily that the industry almost collapsed, and most sponge-like materials are now synthetic. Sponges and their microscopic endosymbionts are now being researched as possible sources of medicines for treating a wide range of diseases. [[Dolphin]]s have been observed using sponges as tools while [[foraging]].<ref name="Krutzen 2005" /> |
|||
== Distinguishing features == |
== Distinguishing features == |
||
{{Further|Cnidaria|Ctenophore}} |
{{Further|Cnidaria|Ctenophore}} |
||
Sponges constitute the [[phylum]] Porifera, and have been defined as [[Sessility (motility)|sessile]] [[metazoa]]ns (multicelled immobile animals) that have water intake and outlet openings connected by chambers lined with [[choanocyte]]s, cells with whip-like flagella.{{sfn|Bergquist|1978|p=29}} However, a few carnivorous sponges have lost these water flow systems and the choanocytes.{{sfn|Bergquist|1978|p=39}}<ref name="HooperVanSoestDebrenne2002PhylumPorifera">{{cite book| vauthors = Hooper JN, Van Soest RW, Debrenne F |contribution=Phylum Porifera Grant, 1836|pages=9–14| veditors = Hooper JN, Van Soest RW |title=Systema Porifera: A Guide to the Classification of Sponges|publisher=Kluwer Academic/Plenum|location=New York|url={{google books |plainurl=y |id=OQoxzqjQXWEC|page=220}}|year=2002 |isbn=978-0-306-47260-2}}</ref> All known living sponges can remold their bodies, as most types of their cells can move within their bodies and a few can change from one type to another.<ref name="HooperVanSoestDebrenne2002PhylumPorifera" /><ref name="RuppertBarnes2004Porifera" /> |
|||
Sponges constitute the [[phylum]] Porifera, and have been defined as [[Sessility (motility)|sessile]] [[metazoa]]ns (multicelled immobile animals) that have water intake and outlet openings connected by chambers lined with [[choanocyte]]s, cells with whip-like flagella.<ref name="Bergquist_1978"/>{{rp|29}} However, a few carnivorous sponges have lost these water flow systems and the choanocytes.<ref name="Bergquist_1978"/>{{rp|39}}<ref name="Hooper_2002">{{cite book |author1=Hooper, J. N. A. |author2=Van Soest, R. W. M. |author3=Debrenne, F. |contribution=Phylum Porifera Grant, 1836|pages=9–14 |editor1=Hooper, J. N. A. |editor2=Van Soest, R. W. M. |title=Systema Porifera: A Guide to the Classification of Sponges |publisher=Kluwer Academic/Plenum |location=New York |url={{google books |plainurl=y |id=OQoxzqjQXWEC|page=220}}|year=2002 |isbn=978-0-306-47260-2}}</ref> All known living sponges can remold their bodies, as most types of their cells can move within their bodies and a few can change from one type to another.<ref name="Hooper_2002"/><ref name="Ruppert_2004">{{cite book |last1=Ruppert |first1=Edward E. |last2=Fox |first2=Richard S. |last3=Barnes |first3=Robert D. |title=Invertebrate Zoology |publisher=Brooks / [[COLE Publishing]] |edition=7th |isbn=978-0-03-025982-1 |year=2004 |url-access=registration |url=https://archive.org/details/isbn_9780030259821 |pages=76–97}}</ref> |
|||
Even if a few sponges are able to produce mucus – which acts as a microbial barrier in all other animals – no sponge with the ability to secrete a functional mucus layer has been recorded. Without such a mucus layer their living tissue is covered by a layer of microbial symbionts, which can contribute up to 40–50% of the sponge wet mass. This inability to prevent microbes from penetrating their porous tissue could be a major reason why they have never evolved a more complex anatomy.<ref>{{cite journal | vauthors = Bakshani CR, Morales-Garcia AL, Althaus M, Wilcox MD, Pearson JP, Bythell JC, Burgess JG | title = Evolutionary conservation of the antimicrobial function of mucus: a first defence against infection | language = En | journal = npj Biofilms and Microbiomes | volume = 4 | issue = 1 | page = 14 | date = 2018-07-04 | pmid = 30002868 | pmc = 6031612 | doi = 10.1038/s41522-018-0057-2 }}</ref> |
|||
Even if a few sponges are able to produce mucus – which acts as a microbial barrier in all other animals – no sponge with the ability to secrete a functional mucus layer has been recorded. Without such a mucus layer their living tissue is covered by a layer of microbial symbionts, which can contribute up to 40–50% of the sponge wet mass. This inability to prevent microbes from penetrating their porous tissue could be a major reason why they have never evolved a more complex anatomy.<ref>{{cite journal |last1=Bakshani |first1=Cassie R. |last2=Morales-Garcia |first2=Ana L. |last3=Althaus |first3=Mike |last4=Wilcox |first4=Matthew D. |last5=Pearson |first5=Jeffrey P. |last6=Bythell |first6=John C. |last7=Burgess |first7=J. Grant |title=Evolutionary conservation of the antimicrobial function of mucus: a first defence against infection |journal=npj Biofilms and Microbiomes |volume=4 |issue=1 |page=14 |date=2018-07-04 |pmid=30002868 |pmc=6031612 |doi=10.1038/s41522-018-0057-2 }}</ref> |
|||
Like [[cnidaria]]ns (jellyfish, etc.) and [[Ctenophora|ctenophores]] (comb jellies), and unlike all other known metazoans, sponges' bodies consist of a non-living jelly-like mass ([[mesohyl]]) sandwiched between two main layers of cells.<ref name="Bergquist2001PoriferaInAnderson" /><ref name="Hinde2001CnidariaAndCtenophoraInAnderson">{{cite book| vauthors = Hinde RT |year=1998|chapter=The Cnidaria and Ctenophora|pages=28–57| veditors = Anderson DT |title=Invertebrate Zoology|publisher=[[Oxford University Press]]|isbn=978-0-19-551368-4}}</ref> Cnidarians and ctenophores have simple nervous systems, and their cell layers are bound by internal connections and by being mounted on a basement membrane (thin fibrous mat, also known as "[[basal lamina]]").<ref name="Hinde2001CnidariaAndCtenophoraInAnderson" /> Sponges do not have a nervous system similar to that of vertebrates but may have one that is quite different.<ref name="nerve1"/> Their middle jelly-like layers have large and varied populations of cells, and some types of cells in their outer layers may move into the middle layer and change their functions.<ref name="RuppertBarnes2004Porifera" /> |
|||
Like [[cnidaria]]ns (jellyfish, etc.) and [[Ctenophora|ctenophores]] (comb jellies), and unlike all other known metazoans, sponges' bodies consist of a non-living jelly-like mass ([[mesohyl]]) sandwiched between two main layers of cells.<ref name="Bergquist_1998"/><ref name="Hinde_2001">{{cite book |last=Hinde |first=R. T. |year=1998 |chapter=The Cnidaria and Ctenophora|pages=28–57 |editor-last=Anderson |editor-first=D. T. |title=Invertebrate Zoology |publisher=[[Oxford University Press]] |isbn=978-0-19-551368-4}}</ref> Cnidarians and ctenophores have simple nervous systems, and their cell layers are bound by internal connections and by being mounted on a basement membrane (thin fibrous mat, also known as "[[basal lamina]]").<ref name="Hinde_2001"/> Sponges do not have a nervous system similar to that of vertebrates but may have one that is quite different.<ref name="Moroz_2022"/> Their middle jelly-like layers have large and varied populations of cells, and some types of cells in their outer layers may move into the middle layer and change their functions.<ref name="Ruppert_2004"/> |
|||
{| class="wikitable" <!-- width="45%" --> style="margin-left:4px" |
|||
{|class="wikitable" <!-- width="45%" --> style="margin-left:4px" |
|||
|- |
|- |
||
! !! Sponges<ref name=" |
! !! Sponges<ref name="Ruppert_2004"/><ref name="Bergquist_1998"/> !! [[Cnidarian]]s and [[ctenophore]]s<ref name="Hinde_2001"/> |
||
|- |
|- |
||
! Nervous system |
! Nervous system |
||
| |
|No/Yes ||Yes, simple |
||
|- |
|- |
||
! Cells in each layer bound together |
! Cells in each layer bound together |
||
| |
|No, except that [[Homoscleromorpha]] have basement membranes.<ref name="Exposito_2002"/> ||Yes: inter-cell connections; basement membranes |
||
|- |
|- |
||
! Number of cells in middle "jelly" layer |
! Number of cells in middle "jelly" layer |
||
| |
|Many ||Few |
||
|- |
|- |
||
! Cells in outer layers can move inwards and change functions |
! Cells in outer layers can move inwards and change functions |
||
| |
|Yes ||No |
||
|} |
|} |
||
| Line 101: | Line 77: | ||
=== Cell types === |
=== Cell types === |
||
{{anchor|Ostium (sponges)}} |
{{anchor|Ostium (sponges)}} |
||
{{Annotated image/Porifera cell types}} |
{{Annotated image/Porifera cell types}} |
||
A sponge's body is hollow and is held in shape by the [[mesohyl]], a jelly-like substance made mainly of [[collagen]] and reinforced by a dense network of fibers also made of collagen. The inner surface is covered with [[choanocyte]]s, cells with cylindrical or conical collars surrounding one [[flagellum]] per choanocyte. The wave-like motion of the whip-like flagella drives water through the sponge's body. All sponges have '''ostia''', channels leading to the interior through the mesohyl, and in most sponges these are controlled by tube-like [[porocytes]] that form closable inlet valves. [[Pinacocyte]]s, plate-like cells, form a single-layered external skin over all other parts of the mesohyl that are not covered by choanocytes, and the pinacocytes also digest food particles that are too large to enter the ostia,<ref name=" |
A sponge's body is hollow and is held in shape by the [[mesohyl]], a jelly-like substance made mainly of [[collagen]] and reinforced by a dense network of fibers also made of collagen. 18 distinct cell types have been identified.<ref>{{cite journal |last1=Musser |first1=Jacob M. |last2=Schippers |first2=Klaske J. |last3=Nickel |first3=Michael |last4=Mizzon |first4=Giulia |last5=Kohn |first5=Andrea B. |last6=Pape |first6=Constantin |last7=Ronchi |first7=Paolo |last8=Papadopoulos |first8=Nikolaos |last9=Tarashansky |first9=Alexander J. |last10=Hammel |first10=Jörg U. |last11=Wolf |first11=Florian |last12=Liang |first12=Cong |last13=Hernández-Plaza |first13=Ana |last14=Cantalapiedra |first14=Carlos P. |last15=Achim |first15=Kaia |last16=Schieber |first16=Nicole L. |last17=Pan |first17=Leslie |last18=Ruperti |first18=Fabian |last19=Francis |first19=Warren R. |last20=Vargas |first20=Sergio |last21=Kling |first21=Svenja |last22=Renkert |first22=Maike |last23=Polikarpov |first23=Maxim |last24=Bourenkov |first24=Gleb |last25=Feuda |first25=Roberto |last26=Gaspar |first26=Imre |last27=Burkhardt |first27=Pawel |last28=Wang |first28=Bo |last29=Bork |first29=Peer |last30=Beck |first30=Martin |last31=Schneider |first31=Thomas R. |last32=Kreshuk |first32=Anna |last33=Wörheide |first33=Gert |last34=Huerta-Cepas |first34=Jaime |last35=Schwab |first35=Yannick |last36=Moroz |first36=Leonid L. |last37=Arendt |first37=Detlev |display-authors=6 |title=Profiling cellular diversity in sponges informs animal cell type and nervous system evolution |journal=Science |volume=374 |issue=6568 |pages=717–723 |date=November 2021 |pmid=34735222 |pmc=9233960 |doi=10.1126/science.abj2949 |bibcode=2021Sci...374..717M }}</ref> The inner surface is covered with [[choanocyte]]s, cells with cylindrical or conical collars surrounding one [[flagellum]] per choanocyte. The wave-like motion of the whip-like flagella drives water through the sponge's body. All sponges have '''ostia''', channels leading to the interior through the mesohyl, and in most sponges these are controlled by tube-like [[porocytes]] that form closable inlet valves. [[Pinacocyte]]s, plate-like cells, form a single-layered external skin over all other parts of the mesohyl that are not covered by choanocytes, and the pinacocytes also digest food particles that are too large to enter the ostia,<ref name="Ruppert_2004"/><ref name="Bergquist_1998"/> while those at the base of the animal are responsible for anchoring it.<ref name="Bergquist_1998"/> |
||
Other types of cells live and move within the mesohyl:<ref name="Ruppert_2004"/><ref name="Bergquist_1998"/> |
|||
Other types of cells live and move within the mesohyl:<ref name="RuppertBarnes2004Porifera" /><ref name="Bergquist2001PoriferaInAnderson" /> |
|||
* [[Lophocyte]]s are [[amoeba]]-like cells that move slowly through the mesohyl and secrete collagen fibres. |
* [[Lophocyte]]s are [[amoeba]]-like cells that move slowly through the mesohyl and secrete collagen fibres. |
||
* [[Collencyte]]s are another type of collagen-producing cell. |
* [[Collencyte]]s are another type of collagen-producing cell. |
||
| Line 116: | Line 94: | ||
* [[Archaeocytes]] (or [[amoebocytes]]) are [[amoeba]]-like cells that are [[totipotent]], in other words, each is capable of transformation into any other type of cell. They also have important roles in feeding and in clearing debris that block the ostia. |
* [[Archaeocytes]] (or [[amoebocytes]]) are [[amoeba]]-like cells that are [[totipotent]], in other words, each is capable of transformation into any other type of cell. They also have important roles in feeding and in clearing debris that block the ostia. |
||
Many larval sponges possess neuron-less [[eye]]s that are based on [[cryptochrome]]s. They mediate phototaxic behavior.<ref>{{cite journal | |
Many larval sponges possess neuron-less [[eye]]s that are based on [[cryptochrome]]s. They mediate phototaxic behavior.<ref>{{cite journal |last1=Rivera |first1=A.S. |last2=Ozturk |first2=N. |last3=Fahey |first3=B. |last4=Plachetzki |first4=D.C. |last5=Degnan |first5=B.M. |last6=Sancar |first6=A. |last7=Oakley |first7=T.H. |title=Blue-light-receptive cryptochrome is expressed in a sponge eye lacking neurons and opsin |journal=The Journal of Experimental Biology |volume=215 |issue=Pt 8 |pages=1278–86 |date=April 2012 |pmid=22442365 |pmc=3309880 |doi=10.1242/jeb.067140 }}</ref> |
||
[[Glass sponge]]s present a distinctive variation on this basic plan. Their spicules, which are made of [[silica]], form a [[scaffolding]]-like framework between whose rods the living tissue is suspended like a [[Spider web|cobweb]] that contains most of the cell types.<ref name="Ruppert_2004"/> This tissue is a [[Hexactinellid#Syncytia|syncytium]] that in some ways behaves like many cells that share a single external [[Cell membrane|membrane]], and in others like a single cell with multiple [[cell nucleus|nuclei]]. |
|||
=== Glass sponges' syncytia === |
|||
[[File:Euplectella 01.png|thumb|left|The [[glass sponge]] ''[[Euplectella]]''.{{sfn|Ruppert|Fox|Barnes|2004|p=83|loc=Fig. 5-7}} |
|||
{{legend|#4848ff|Water flow}} |
|||
{{legend|#c0c0c0|Main [[syncitium]]}} |
|||
{{legend|#6a6a6a|[[Sponge spicule|Spicules]]}} |
|||
{{legend|red|[[Choanosyncitium]] and collar bodies showing interior}} |
|||
]] |
|||
[[Glass sponge]]s present a distinctive variation on this basic plan. Their spicules, which are made of [[silica]], form a [[scaffolding]]-like framework between whose rods the living tissue is suspended like a [[Spider web|cobweb]] that contains most of the cell types.<ref name="RuppertBarnes2004Porifera" /> This tissue is a [[syncytium]] that in some ways behaves like many cells that share a single external [[Cell membrane|membrane]], and in others like a single cell with multiple [[cell nucleus|nuclei]]. The mesohyl is absent or minimal. The syncytium's [[cytoplasm]], the soupy fluid that fills the interiors of cells, is organized into "rivers" that transport nuclei, [[organelle]]s ("organs" within cells) and other substances.<ref name="Leys2003SignificanceOfSyncytialTissues">{{cite journal | vauthors = Leys SP | title = The significance of syncytial tissues for the position of the hexactinellida in the metazoa | journal = Integrative and Comparative Biology | volume = 43 | issue = 1 | pages = 19–27 | date = February 2003 | pmid = 21680406 | doi = 10.1093/icb/43.1.19 | doi-access = free }}</ref> Instead of choanocytes, they have further syncytia, known as choanosyncytia, which form bell-shaped chambers where water enters via perforations. The insides of these chambers are lined with "collar bodies", each consisting of a collar and flagellum but without a nucleus of its own. The motion of the flagella sucks water through passages in the "cobweb" and expels it via the open ends of the bell-shaped chambers.<ref name="RuppertBarnes2004Porifera" /> |
|||
=== Water flow and body structures === |
|||
Some types of cells have a single nucleus and membrane each but are connected to other single-nucleus cells and to the main syncytium by "bridges" made of [[cytoplasm]]. The [[sclerocyte]]s that build spicules have multiple nuclei, and in glass sponge larvae they are connected to other tissues by cytoplasm bridges; such connections between sclerocytes have not so far been found in adults, but this may simply reflect the difficulty of investigating such small-scale features. The bridges are controlled by "plugged junctions" that apparently permit some substances to pass while blocking others.<ref name="Leys2003SignificanceOfSyncytialTissues" /> |
|||
{{Clear}} |
|||
{{Annotated image |float={{{float|right}}} |caption=[[Porifera]] body structures<ref>{{cite book |
|||
|last1=Ruppert|first1=Edward E.|last2=Fox|first2=Richard S.|last3=Barnes|first3=Robert D. |title=Invertebrate Zoology |publisher=Brooks / Cole |edition=7th |isbn=978-0-03-025982-1 |date=2004 |page=78 |ref=none}}</ref> |image=Porifera body structures 01.png |width=280 |image-width=280 |height=205 |
|||
{{Annotated image | float={{{float|right}}} | caption=[[Porifera]] body structures<ref>{{cite book |
|||
|annotations= |
|||
| vauthors = Ruppert EE, Fox RS, Barnes RD |
|||
| title=Invertebrate Zoology |
|||
| publisher=Brooks / Cole | edition=7th | isbn=978-0-03-025982-1 | date=2004 | page=78 | ref=none |
|||
}}</ref> | image=Porifera body structures 01.png | width=280 | image-width=280 | height=205 |
|||
| annotations= |
|||
{{Annotation|5|150|'''Asconoid'''}} |
{{Annotation|5|150|'''Asconoid'''}} |
||
{{Annotation|105|150|'''Syconoid'''}} |
{{Annotation|105|150|'''Syconoid'''}} |
||
| Line 146: | Line 112: | ||
}} |
}} |
||
Most sponges work rather like [[chimney]]s: they take in water at the bottom and eject it from the [[osculum]] |
Most sponges work rather like [[chimney]]s: they take in water at the bottom and eject it from the [[osculum]] at the top. Since ambient currents are faster at the top, the suction effect that they produce by [[Bernoulli's principle]] does some of the work for free. Sponges can control the water flow by various combinations of wholly or partially closing the osculum and ostia (the intake pores) and varying the beat of the flagella, and may shut it down if there is a lot of sand or silt in the water.<ref name="Ruppert_2004"/> |
||
Although the layers of [[pinacocyte]]s and [[choanocyte]]s resemble the [[epithelia]] of more complex animals, they are not bound tightly by cell-to-cell connections or a basal lamina (thin fibrous sheet underneath). The flexibility of these layers and re-modeling of the mesohyl by lophocytes allow the animals to adjust their shapes throughout their lives to take maximum advantage of local water currents.{{ |
Although the layers of [[pinacocyte]]s and [[choanocyte]]s resemble the [[epithelia]] of more complex animals, they are not bound tightly by cell-to-cell connections or a basal lamina (thin fibrous sheet underneath). The flexibility of these layers and re-modeling of the mesohyl by lophocytes allow the animals to adjust their shapes throughout their lives to take maximum advantage of local water currents.<ref name="Ruppert_2004"/>{{rp|83}} |
||
The simplest body structure in sponges is a tube or vase shape known as "asconoid", but this severely limits the size of the animal. The body structure is characterized by a stalk-like spongocoel surrounded by a single layer of choanocytes. If it is simply scaled up, the ratio of its volume to surface area increases, because surface increases as the square of length or width while volume increases proportionally to the cube. The amount of tissue that needs food and [[oxygen]] is determined by the volume, but the pumping capacity that supplies food and oxygen depends on the area covered by choanocytes. Asconoid sponges seldom exceed {{convert|1|mm|in|abbr=on}} in diameter.<ref name=" |
The simplest body structure in sponges is a tube or vase shape known as "asconoid", but this severely limits the size of the animal. The body structure is characterized by a stalk-like spongocoel surrounded by a single layer of choanocytes. If it is simply scaled up, the ratio of its volume to surface area increases, because surface increases as the square of length or width while volume increases proportionally to the cube. The amount of tissue that needs food and [[oxygen]] is determined by the volume, but the pumping capacity that supplies food and oxygen depends on the area covered by choanocytes. Asconoid sponges seldom exceed {{convert|1|mm|in|abbr=on}} in diameter.<ref name="Ruppert_2004"/> |
||
[[File:Sea sponge diagram.svg|thumb|250px|left|Diagram of a syconoid sponge]] |
[[File:Sea sponge diagram.svg|thumb|250px|left|Diagram of a syconoid sponge]] |
||
| Line 156: | Line 122: | ||
Some sponges overcome this limitation by adopting the "syconoid" structure, in which the body wall is [[pleat]]ed. The inner pockets of the pleats are lined with choanocytes, which connect to the outer pockets of the pleats by ostia. This increase in the number of choanocytes and hence in pumping capacity enables syconoid sponges to grow up to a few centimeters in diameter. |
Some sponges overcome this limitation by adopting the "syconoid" structure, in which the body wall is [[pleat]]ed. The inner pockets of the pleats are lined with choanocytes, which connect to the outer pockets of the pleats by ostia. This increase in the number of choanocytes and hence in pumping capacity enables syconoid sponges to grow up to a few centimeters in diameter. |
||
{{anchor|leuconoid}}The "leuconoid" pattern boosts pumping capacity further by filling the interior almost completely with mesohyl that contains a network of chambers lined with choanocytes and connected to each other and to the water intakes and outlet by tubes. Leuconid sponges grow to over {{convert|1|m|ft|abbr=on}} in diameter, and the fact that growth in any direction increases the number of choanocyte chambers enables them to take a wider range of forms, for example, "encrusting" sponges whose shapes follow those of the surfaces to which they attach. All freshwater and most shallow-water marine sponges have leuconid bodies. The networks of water passages in [[glass sponge]]s are similar to the leuconid structure.<ref name=" |
{{anchor|leuconoid}}The "leuconoid" pattern boosts pumping capacity further by filling the interior almost completely with mesohyl that contains a network of chambers lined with choanocytes and connected to each other and to the water intakes and outlet by tubes. Leuconid sponges grow to over {{convert|1|m|ft|abbr=on}} in diameter, and the fact that growth in any direction increases the number of choanocyte chambers enables them to take a wider range of forms, for example, "encrusting" sponges whose shapes follow those of the surfaces to which they attach. All freshwater and most shallow-water marine sponges have leuconid bodies. The networks of water passages in [[glass sponge]]s are similar to the leuconid structure.<ref name="Ruppert_2004"/> |
||
In all three types of structure the cross-section area of the choanocyte-lined regions is much greater than that of the intake and outlet channels. This makes the flow slower near the choanocytes and thus makes it easier for them to trap food particles.<ref name="RuppertBarnes2004Porifera" /> For example, in ''[[Leuconia]]'', a small leuconoid sponge about {{convert|10|cm|in}} tall and {{convert|1|cm|in}} in diameter, water enters each of more than 80,000 intake canals at 6 cm per ''minute''. However, because ''Leuconia'' has more than 2 million flagellated chambers whose combined diameter is much greater than that of the canals, water flow through chambers slows to 3.6 cm per ''hour'', making it easy for choanocytes to capture food. All the water is expelled through a single [[osculum]] at about 8.5 cm per ''second'', fast enough to carry waste products some distance away.<ref>{{cite book| vauthors = Hickman CP, Roberts LS, Larson A |year=2001|title=Integrated Principles of Zoology|edition=11th|publisher=[[McGraw-Hill]]|location=New York|isbn=978-0-07-290961-6|page=247}}</ref> |
|||
In all three types of structure the cross-section area of the choanocyte-lined regions is much greater than that of the intake and outlet channels. This makes the flow slower near the choanocytes and thus makes it easier for them to trap food particles.<ref name="Ruppert_2004"/> For example, in ''[[Leuconia]]'', a small leuconoid sponge about {{convert|10|cm|in}} tall and {{convert|1|cm|in}} in diameter, water enters each of more than 80,000 intake canals at 6 cm per ''minute''. However, because ''Leuconia'' has more than 2 million flagellated chambers whose combined diameter is much greater than that of the canals, water flow through chambers slows to 3.6 cm per ''hour'', making it easy for choanocytes to capture food. All the water is expelled through a single [[osculum]] at about 8.5 cm per ''second'', fast enough to carry waste products some distance away.<ref>{{cite book |author1=Hickman, C.P. |author2=Roberts, L.S. |author3=Larson, A. |year=2001 |title=Integrated Principles of Zoology |edition=11th |publisher=[[McGraw-Hill]] |location=New York |isbn=978-0-07-290961-6 |page=247}}</ref> |
|||
[[File:Porifera calcifying 01.png|thumb|Sponge with calcium carbonate skeleton.<ref name=" |
[[File:Porifera calcifying 01.png|thumb|Sponge with calcium carbonate skeleton.<ref name="Ruppert_2004"/> |
||
{{legend-col |
{{legend-col |
||
|{{legend|yellow|[[Pinacocyte]]}} |
|{{legend|yellow|[[Pinacocyte]]}} |
||
| Line 173: | Line 140: | ||
=== Skeleton === |
=== Skeleton === |
||
In zoology a [[skeleton]] is any fairly rigid structure of an animal, irrespective of whether it has joints and irrespective of whether it is [[biomineralization|biomineralized]]. The mesohyl functions as an [[endoskeleton]] in most sponges, and is the only skeleton in soft sponges that encrust hard surfaces such as rocks. More commonly the mesohyl is stiffened by mineral [[sponge spicule|spicules]], by spongin fibers or both. Spicules, which are present in most but not all species,<ref>{{Cite web|url=http://species-identification.org/species.php?species_group=sponges&id=259|title=Marine Species Identification Portal: Halisarca dujardini|website=species-identification.org|access-date=2019-08-02|archive-date=2020-10-17|archive-url=https://web.archive.org/web/20201017130847/http://species-identification.org/species.php?species_group=sponges&id=259|url-status=dead}}</ref> may be made of [[silica]] or calcium carbonate, and vary in shape from simple rods to three-dimensional "stars" with up to six rays. Spicules are produced by [[sclerocyte]] cells,<ref name="RuppertBarnes2004Porifera" /> and may be separate, connected by joints, or fused.<ref name="HooperVanSoestDebrenne2002PhylumPorifera" /> |
|||
In zoology a [[skeleton]] is any fairly rigid structure of an animal, irrespective of whether it has joints and irrespective of whether it is [[biomineralization|biomineralized]]. The mesohyl functions as an [[endoskeleton]] in most sponges, and is the only skeleton in soft sponges that encrust hard surfaces such as rocks. More commonly the mesohyl is stiffened by mineral [[sponge spicule|spicules]], by spongin fibers or both. Spicules, which are present in most but not all species,<ref>{{Cite web |url=http://species-identification.org/species.php?species_group=sponges&id=259 |title=Marine Species Identification Portal: Halisarca dujardini|website=species-identification.org |access-date=2019-08-02 |archive-date=2020-10-17 |archive-url=https://web.archive.org/web/20201017130847/http://species-identification.org/species.php?species_group=sponges&id=259 |url-status=dead}}</ref> may be made of [[silica]] or calcium carbonate, and vary in shape from simple rods to three-dimensional "stars" with up to six rays. Spicules are produced by [[sclerocyte]] cells,<ref name="Ruppert_2004"/> and may be separate, connected by joints, or fused.<ref name="Hooper_2002"/> |
|||
Some sponges also secrete [[exoskeleton]]s that lie completely outside their organic components. For example, [[sclerosponge]]s ("hard sponges") have massive calcium carbonate exoskeletons over which the organic matter forms a thin layer with [[choanocyte]] chambers in pits in the mineral. These exoskeletons are secreted by the [[pinacocyte]]s that form the animals' skins.<ref name="RuppertBarnes2004Porifera" /> |
|||
{{Clear}} |
|||
Some sponges also secrete [[exoskeleton]]s that lie completely outside their organic components. For example, [[sclerosponge]]s ("hard sponges") have massive calcium carbonate exoskeletons over which the organic matter forms a thin layer with [[choanocyte]] chambers in pits in the mineral. These exoskeletons are secreted by the [[pinacocyte]]s that form the animals' skins.<ref name="Ruppert_2004"/> |
|||
== Vital functions == |
== Vital functions == |
||
[[File:Spongia officinalis.jpg|thumb|right|''[[Spongia officinalis]]'', "the kitchen sponge", is dark grey when alive.]] |
[[File:Spongia officinalis.jpg|thumb|right|''[[Spongia officinalis]]'', "the kitchen sponge", is dark grey when alive.]] |
||
=== Movement === |
=== Movement === |
||
Although adult sponges are fundamentally [[Sessility (zoology)|sessile]] animals, some marine and freshwater species can move across the sea bed at speeds of {{convert|1|-|4|mm|in|abbr=on}} per day, as a result of [[amoeba]]-like movements of [[pinacocyte]]s and other cells. A few species can contract their whole bodies, and many can close their [[osculum|oscula]] and [[wikt:ostium|ostia]]. Juveniles drift or swim freely, while adults are stationary.<ref name="RuppertBarnes2004Porifera" /> |
|||
Although adult sponges are fundamentally [[Sessility (zoology)|sessile]] animals, some marine and freshwater species can move across the sea bed at speeds of {{convert|1|-|4|mm|in|abbr=on}} per day, as a result of [[amoeba]]-like movements of [[pinacocyte]]s and other cells. A few species can contract their whole bodies, and many can close their [[osculum|oscula]] and [[wikt:ostium|ostia]]. Juveniles drift or swim freely, while adults are stationary.<ref name="Ruppert_2004"/> |
|||
=== Respiration, feeding and excretion === |
=== Respiration, feeding and excretion === |
||
[[File:Venus Flower Basket.jpg|thumb|right|''[[Euplectella aspergillum]]'', a [[glass sponge]] known as "Venus' flower basket"]] |
|||
[[File:Venus Flower Basket.jpg|thumb|''[[Euplectella aspergillum]]'', a [[glass sponge]] known as "Venus's flower basket"]] |
|||
Sponges do not have distinct [[circulatory]], [[respiratory]], [[digestion|digestive]], and [[excretory]] systems – instead, the water flow system supports all these functions. They [[filter feeding|filter]] food particles out of the water flowing through them. Particles larger than 50 micrometers cannot enter the [[wikt:ostium|ostia]] and [[pinacocyte]]s consume them by [[phagocytosis]] (engulfing and intracellular digestion). Particles from 0.5 μm to 50 μm are trapped in the ostia, which taper from the outer to inner ends. These particles are consumed by pinacocytes or by [[archaeocyte]]s which partially extrude themselves through the walls of the ostia. Bacteria-sized particles, below 0.5 micrometers, pass through the ostia and are caught and consumed by [[choanocyte]]s.<ref name="RuppertBarnes2004Porifera" /> Since the smallest particles are by far the most common, choanocytes typically capture 80% of a sponge's food supply.<ref name="Bergquist2001InEncOfLifeSci">{{cite book|contribution=Porifera (Sponges)| vauthors = Bergquist PR |title=Encyclopedia of Life Sciences|year=2001|publisher= John Wiley & Sons, Ltd.|doi=10.1038/npg.els.0001582|isbn=978-0-470-01617-6}}</ref> Archaeocytes transport food packaged in [[Vesicle (biology)|vesicles]] from cells that directly digest food to those that do not. At least one species of sponge has internal fibers that function as tracks for use by nutrient-carrying archaeocytes,<ref name="RuppertBarnes2004Porifera" /> and these tracks also move inert objects.<ref name="Bergquist2001PoriferaInAnderson" /> |
|||
Sponges do not have distinct [[circulatory]], [[respiratory]], [[digestion|digestive]], and [[excretory]] systems – instead, the water flow system supports all these functions. They [[filter feeding|filter]] food particles out of the water flowing through them. Particles larger than 50 micrometers cannot enter the [[wikt:ostium|ostia]] and [[pinacocyte]]s consume them by [[phagocytosis]] (engulfing and intracellular digestion). Particles from 0.5 μm to 50 μm are trapped in the ostia, which taper from the outer to inner ends. These particles are consumed by pinacocytes or by [[archaeocyte]]s which partially extrude themselves through the walls of the ostia. Bacteria-sized particles, below 0.5 micrometers, pass through the ostia and are caught and consumed by [[choanocyte]]s.<ref name="Ruppert_2004"/> Since the smallest particles are by far the most common, choanocytes typically capture 80% of a sponge's food supply.<ref name="Bergquist_2001">{{cite book |contribution=Porifera (Sponges) |last=Bergquist |first=P.R. |title=Encyclopedia of Life Sciences |year=2001 |publisher= John Wiley & Sons |doi=10.1038/npg.els.0001582 |isbn=978-0-470-01617-6}}</ref> Archaeocytes transport food packaged in [[Vesicle (biology)|vesicles]] from cells that directly digest food to those that do not. At least one species of sponge has internal fibers that function as tracks for use by nutrient-carrying archaeocytes,<ref name="Ruppert_2004"/> and these tracks also move inert objects.<ref name="Bergquist_1998"/> |
|||
It used to be claimed that [[glass sponge]]s could live on nutrients dissolved in sea water and were very averse to silt.<ref name="Krautter1998EcologyOfSiliceousSponges">{{cite journal| vauthors = Krautter M |title=Ecology of siliceous sponges: Application to the environmental interpretation of the Upper Jurassic sponge facies (Oxfordian) from Spain |journal=[[Journal of Iberian Geology|Cuadernos de Geología Ibérica]] |pages=223–239 |year=1998 |volume=24 |url=http://www.ucm.es/BUCM/revistas/geo/16986180/articulos/JIGE9898110223A.PDF |archive-url=https://web.archive.org/web/20090319205858/http://www.ucm.es/BUCM/revistas/geo/16986180/articulos/JIGE9898110223A.PDF |archive-date=March 19, 2009 |access-date=2008-10-10 |url-status=dead }}</ref> However, a study in 2007 found no evidence of this and concluded that they extract bacteria and other micro-organisms from water very efficiently (about 79%) and process suspended sediment grains to extract such prey.<ref>{{cite journal|doi=10.4319/lo.2007.52.1.0428| vauthors = Yahel G, Whitney F, Reiswig HM, Eerkes-Medrano DI, Leys SP |year=2007|title=In situ feeding and metabolism of glass sponges (Hexactinellida, Porifera) studied in a deep temperate fjord with a remotely operated submersible|journal=[[Limnology and Oceanography]]|volume=52|issue=1|pages=428–440|citeseerx=10.1.1.597.9627|bibcode=2007LimOc..52..428Y| s2cid = 86297053 }}</ref> Collar bodies digest food and distribute it wrapped in vesicles that are transported by [[dynein]] "motor" molecules along bundles of [[microtubule]]s that run throughout the [[syncytium]].<ref name="RuppertBarnes2004Porifera" /> |
|||
It used to be claimed that [[glass sponge]]s could live on nutrients dissolved in sea water and were very averse to silt.<ref name="Krautter_1998">{{cite journal |last=Krautter |first=M. |title=Ecology of siliceous sponges: Application to the environmental interpretation of the Upper Jurassic sponge facies (Oxfordian) from Spain |journal=[[Journal of Iberian Geology|Cuadernos de Geología Ibérica]] |pages=223–239 |year=1998 |volume=24 |url=http://www.ucm.es/BUCM/revistas/geo/16986180/articulos/JIGE9898110223A.PDF |archive-url=https://web.archive.org/web/20090319205858/http://www.ucm.es/BUCM/revistas/geo/16986180/articulos/JIGE9898110223A.PDF |archive-date=March 19, 2009 |access-date=2008-10-10 |url-status=dead }}</ref> However, a study in 2007 found no evidence of this and concluded that they extract bacteria and other micro-organisms from water very efficiently (about 79%) and process suspended sediment grains to extract such prey.<ref>{{cite journal |doi=10.4319/lo.2007.52.1.0428 |author1=Yahel, G. |author2=Whitney, F. |author3=Reiswig, H.M. |author4=Eerkes-Medrano, D.I. |author5=Leys, S.P. |year=2007 |title=In situ feeding and metabolism of glass sponges (Hexactinellida, Porifera) studied in a deep temperate fjord with a remotely operated submersible |journal=[[Limnology and Oceanography]] |volume=52|issue=1|pages=428–440|citeseerx=10.1.1.597.9627|bibcode=2007LimOc..52..428Y|s2cid=86297053 }}</ref> Collar bodies digest food and distribute it wrapped in vesicles that are transported by [[dynein]] "motor" molecules along bundles of [[microtubule]]s that run throughout the [[syncytium]].<ref name="Ruppert_2004"/> |
|||
Sponges' cells absorb oxygen by [[diffusion]] from water into cells as water flows through body, into which [[carbon dioxide]] and other soluble waste products such as [[ammonia]] also diffuse. Archeocytes remove mineral particles that threaten to block the ostia, transport them through the mesohyl and generally dump them into the outgoing water current, although some species incorporate them into their skeletons.<ref name="RuppertBarnes2004Porifera" /> |
|||
Sponges' cells absorb oxygen by [[diffusion]] from water into cells as water flows through body, into which [[carbon dioxide]] and other soluble waste products such as [[ammonia]] also diffuse. Archeocytes remove mineral particles that threaten to block the ostia, transport them through the mesohyl and generally dump them into the outgoing water current, although some species incorporate them into their skeletons.<ref name="Ruppert_2004"/> |
|||
=== Carnivorous sponges === |
=== Carnivorous sponges === |
||
[[File:Chondrocladia lampadiglobus.jpg|thumb| The carnivorous ping-pong tree sponge, ''[[Chondrocladia]] lampadiglobus''<ref>{{Cite journal |doi = 10.1371/journal.pone.0035105|title = Global Diversity of Sponges (Porifera)|year = 2012|last1 = Van Soest|first1 = Rob W. M.|last2 = Boury-Esnault|first2 = Nicole|last3 = Vacelet|first3 = Jean|last4 = Dohrmann|first4 = Martin|last5 = Erpenbeck|first5 = Dirk|last6 = De Voogd|first6 = Nicole J.|last7 = Santodomingo|first7 = Nadiezhda|last8 = Vanhoorne|first8 = Bart|last9 = Kelly|first9 = Michelle|author-link9=Michelle Kelly (marine scientist)|last10 = Hooper|first10 = John N. A.|journal = PLOS ONE|volume = 7|issue = 4|pages = e35105|pmid = 22558119|pmc = 3338747|bibcode = 2012PLoSO...735105V|doi-access = free}}</ref>]] |
|||
[[File:Chondrocladia lampadiglobus.jpg|thumb|The carnivorous ping-pong tree sponge, ''[[Chondrocladia]] lampadiglobus''<ref>{{cite journal |last1=Van Soest |first1=Rob W. M. |last2=Boury-Esnault |first2=Nicole |last3=Vacelet |first3=Jean |last4=Dohrmann |first4=Martin |last5=Erpenbeck |first5=Dirk |last6=De Voogd |first6=Nicole J. |last7=Santodomingo |first7=Nadiezhda |last8=Vanhoorne |first8=Bart |last9=Kelly |first9=Michelle |last10=Hooper |first10=John N. A. |title=Global diversity of sponges (Porifera) |journal=PLOS ONE |volume=7 |issue=4 |pages=e35105 |year=2012 |pmid=22558119 |pmc=3338747 |doi=10.1371/journal.pone.0035105 |doi-access=free |bibcode=2012PLoSO...735105V |author-link9=Michelle Kelly (marine scientist) }}</ref>]] |
|||
In waters where the supply of food particles is very poor, some species prey on [[crustacea]]ns and other small animals. So far only 137 species have been discovered.<ref>{{cite news|date=April 19, 2014 |url=http://www.cbc.ca/news/canada/british-columbia/4-new-species-of-killer-sponges-discovered-off-pacific-coast-1.2615509 |title=4 new species of 'killer' sponges discovered off Pacific coast |work=[[CBC News]] |access-date=2014-09-04 |url-status=live |archive-url=https://web.archive.org/web/20140419143139/http://www.cbc.ca/news/canada/british-columbia/4-new-species-of-killer-sponges-discovered-off-pacific-coast-1.2615509 |archive-date=April 19, 2014 }}</ref> Most belong to the [[family (biology)|family]] [[Cladorhizidae]], but a few members of the [[Guitarridae]] and [[Esperiopsidae]] are also carnivores.<ref name="Vacelet2008NewGenusOfCarnivorousSpongesLollipocladia" /> In most cases, little is known about how they actually capture prey, although some species are thought to use either sticky threads or hooked [[sponge spicule|spicules]].<ref name="Vacelet2008NewGenusOfCarnivorousSpongesLollipocladia" /><ref>{{cite journal| vauthors = Watling L |title=Predation on copepods by an Alaskan cladorhizid sponge|journal=[[Journal of the Marine Biological Association of the United Kingdom]]|year= 2007|volume=87|pages=1721–1726|doi=10.1017/S0025315407058560|issue=6|s2cid=86588792}}</ref> Most carnivorous sponges live in deep waters, up to {{convert|8840|m|mi|abbr=on}},<ref name="VaceletBouryEsnault1995CarnivorousSponges">{{cite journal| vauthors = Vacelet J, Boury-Esnault N |title=Carnivorous sponges|journal=Nature|volume=373|pages=333–335|year=1995|doi=10.1038/373333a0|issue=6512|bibcode=1995Natur.373..333V|s2cid=4320216|doi-access=free}}</ref> and the development of deep-ocean exploration techniques is expected to lead to the discovery of several more.<ref name="RuppertBarnes2004Porifera" /><ref name="Vacelet2008NewGenusOfCarnivorousSpongesLollipocladia">{{cite journal| vauthors = Vacelet J |title=A new genus of carnivorous sponges (Porifera: Poecilosclerida, Cladorhizidae) from the deep N-E Pacific, and remarks on the genus ''Neocladia''|journal=[[Zootaxa]]|url=http://www.mapress.com/zootaxa/2008/f/z01752p065f.pdf |archive-url=https://web.archive.org/web/20080906190452/http://www.mapress.com/zootaxa/2008/f/z01752p065f.pdf |archive-date=2008-09-06 |url-status=live|volume=1752|pages=57–65|year=2008|access-date=2008-10-31|doi=10.11646/zootaxa.1752.1.3}}</ref> However, one species has been found in [[Mediterranean]] caves at depths of {{convert|17|-|23|m|ft|abbr=on}}, alongside the more usual [[filter-feeding]] sponges. The cave-dwelling predators capture crustaceans under {{convert|1|mm|in|abbr=on}} long by entangling them with fine threads, digest them by enveloping them with further threads over the course of a few days, and then return to their normal shape; there is no evidence that they use [[venom]].<ref name="VaceletBouryEsnault1995CarnivorousSponges" /> |
|||
In waters where the supply of food particles is very poor, some species prey on [[crustacea]]ns and other small animals. So far only 137 species have been discovered.<ref>{{cite news|date=April 19, 2014 |url=http://www.cbc.ca/news/canada/british-columbia/4-new-species-of-killer-sponges-discovered-off-pacific-coast-1.2615509 |title=4 new species of 'killer' sponges discovered off Pacific coast |work=[[CBC News]] |access-date=2014-09-04 |url-status=live |archive-url=https://web.archive.org/web/20140419143139/http://www.cbc.ca/news/canada/british-columbia/4-new-species-of-killer-sponges-discovered-off-pacific-coast-1.2615509 |archive-date=April 19, 2014 }}</ref> Most belong to the [[family (biology)|family]] [[Cladorhizidae]], but a few members of the [[Guitarridae]] and [[Esperiopsidae]] are also carnivores.<ref name="Vacelet_2008"/> In most cases, little is known about how they actually capture prey, although some species are thought to use either sticky threads or hooked [[sponge spicule|spicules]].<ref name="Vacelet_2008"/><ref>{{cite journal|last= Watling |first=L. |title=Predation on copepods by an Alaskan cladorhizid sponge |journal=[[Journal of the Marine Biological Association of the United Kingdom]] |year= 2007|volume=87 |pages=1721–1726 |doi=10.1017/S0025315407058560|issue=6 |bibcode=2007JMBUK..87.1721W |s2cid=86588792}}</ref> Most carnivorous sponges live in deep waters, up to {{convert|8840|m|mi|abbr=on}},<ref name="Vacelet_1995">{{cite journal |last1= Vacelet |first1=J. |last2=Boury-Esnault |first2=N. |title=Carnivorous sponges|journal=Nature|volume=373|pages=333–335|year=1995|doi=10.1038/373333a0|issue=6512|bibcode=1995Natur.373..333V|s2cid=4320216|doi-access=free}}</ref> and the development of deep-ocean exploration techniques is expected to lead to the discovery of several more.<ref name="Ruppert_2004"/><ref name="Vacelet_2008">{{cite journal |last1=Vacelet |first1=J. |title=A new genus of carnivorous sponges (Porifera: Poecilosclerida, Cladorhizidae) from the deep N-E Pacific, and remarks on the genus ''Neocladia''|journal=[[Zootaxa]]|url=http://www.mapress.com/zootaxa/2008/f/z01752p065f.pdf |archive-url=https://web.archive.org/web/20080906190452/http://www.mapress.com/zootaxa/2008/f/z01752p065f.pdf |archive-date=2008-09-06 |url-status=live|volume=1752|pages=57–65|year=2008|access-date=2008-10-31|doi=10.11646/zootaxa.1752.1.3}}</ref> However, one species has been found in [[Mediterranean]] caves at depths of {{convert|17|-|23|m|ft|abbr=on}}, alongside the more usual [[filter-feeding]] sponges. The cave-dwelling predators capture crustaceans under {{convert|1|mm|in|abbr=on}} long by entangling them with fine threads, digest them by enveloping them with further threads over the course of a few days, and then return to their normal shape; there is no evidence that they use [[venom]].<ref name="Vacelet_1995"/> |
|||
Most known carnivorous sponges have completely lost the water flow system and [[choanocyte]]s. However, the [[genus]] ''[[Chondrocladia]]'' uses a highly modified water flow system to inflate balloon-like structures that are used for capturing prey.<ref name="Vacelet2008NewGenusOfCarnivorousSpongesLollipocladia" /><ref name="VaceletKelly2008carnivorousSpongesEarlyJurassic">{{cite journal| last1=Vacelet |first1=J. |last2=Kelly |first2=M. |author-link2=Michelle Kelly (marine scientist) |title=New species from the deep Pacific suggest that carnivorous sponges date back to the Early Jurassic|journal=Nature Precedings|year=2008|doi= 10.1038/npre.2008.2327.1|doi-access=free}}</ref> |
|||
Most known carnivorous sponges have completely lost the water flow system and [[choanocyte]]s. However, the [[genus]] ''[[Chondrocladia]]'' uses a highly modified water flow system to inflate balloon-like structures that are used for capturing prey.<ref name="Vacelet_2008"/><ref name="Vacele_2008">{{cite journal |last1= Vacelet |first1=J. |last2=Kelly |first2=Michelle |author-link2=Michelle Kelly (marine scientist) |title=New species from the deep Pacific suggest that carnivorous sponges date back to the Early Jurassic|journal=Nature Precedings|year=2008|doi= 10.1038/npre.2008.2327.1|doi-access=free}}</ref> |
|||
=== Endosymbionts === |
=== Endosymbionts === |
||
Freshwater sponges often host [[green algae]] as [[endosymbiont]]s within [[archaeocyte]]s and other cells and benefit from nutrients produced by the algae. Many marine species host other [[photosynthesis|photosynthesizing]] organisms, most commonly [[cyanobacteria]] but in some cases [[dinoflagellate]]s. Symbiotic cyanobacteria may form a third of the total mass of living tissue in some sponges, and some sponges gain 48% to 80% of their energy supply from these micro-organisms.<ref name="RuppertBarnes2004Porifera" /> In 2008, a [[University of Stuttgart]] team reported that spicules made of [[silica]] conduct light into the [[mesohyl]], where the photosynthesizing endosymbionts live.<ref>{{cite journal | vauthors = Brümmer F, Pfannkuchen M, Baltz A, Hauser T, Thiel V | title = Light inside sponges | journal = [[Journal of Experimental Marine Biology and Ecology]] | volume = 367 | issue = 2 | pages = 61–64 | doi = 10.1016/j.jembe.2008.06.036 | year = 2008}} |
|||
*{{cite news |author=Matt Walker |date=10 November 2008 |title=Nature's 'fibre optics' experts |work=BBC News |url=http://news.bbc.co.uk/2/hi/science/nature/7720836.stm}}</ref> Sponges that host photosynthesizing organisms are most common in waters with relatively poor supplies of food particles and often have leafy shapes that maximize the amount of sunlight they collect.<ref name="Bergquist2001PoriferaInAnderson" /> |
|||
Freshwater sponges often host [[green algae]] as [[endosymbiont]]s within [[archaeocyte]]s and other cells and benefit from nutrients produced by the algae. Many marine species host other [[photosynthesis|photosynthesizing]] organisms, most commonly [[cyanobacteria]] but in some cases [[dinoflagellate]]s. Symbiotic cyanobacteria may form a third of the total mass of living tissue in some sponges, and some sponges gain 48% to 80% of their energy supply from these micro-organisms.<ref name="Ruppert_2004"/> In 2008, a [[University of Stuttgart]] team reported that spicules made of [[silica]] conduct light into the [[mesohyl]], where the photosynthesizing endosymbionts live.<ref>{{cite journal |last1=Brümmer |first1=Franz |last2=Pfannkuchen |first2=Martin |last3=Baltz |first3=Alexander |last4=Hauser |first4=Thomas |last5=Thiel |first5=Vera |title=Light inside sponges |journal=[[Journal of Experimental Marine Biology and Ecology]] |volume=367 |issue=2 |pages=61–64 |doi=10.1016/j.jembe.2008.06.036 |year=2008|bibcode=2008JEMBE.367...61B }} |
|||
A recently discovered carnivorous sponge that lives near [[hydrothermal vent]]s hosts [[Methanotrophic|methane-eating]] bacteria and digests some of them.<ref name="Bergquist2001PoriferaInAnderson" /> |
|||
*{{cite news |last=Walker |first=Matt |date=10 November 2008 |title=Nature's 'fibre optics' experts |work=BBC News |url=http://news.bbc.co.uk/2/hi/science/nature/7720836.stm |access-date=11 November 2008 |archive-date=17 December 2008 |archive-url=https://web.archive.org/web/20081217045607/http://news.bbc.co.uk/2/hi/science/nature/7720836.stm |url-status=live }}</ref> Sponges that host photosynthesizing organisms are most common in waters with relatively poor supplies of food particles and often have leafy shapes that maximize the amount of sunlight they collect.<ref name="Bergquist_1998"/> |
|||
A recently discovered carnivorous sponge that lives near [[hydrothermal vent]]s hosts [[Methanotrophic|methane-eating]] bacteria and digests some of them.<ref name="Bergquist_1998"/> |
|||
=== "Immune" system === |
=== "Immune" system === |
||
Sponges do not have the complex [[immune system]]s of most other animals. However, they reject [[Medical grafting|grafts]] from other species but accept them from other members of their own species. In a few marine species, gray cells play the leading role in rejection of foreign material. When invaded, they produce a chemical that stops movement of other cells in the affected area, thus preventing the intruder from using the sponge's internal transport systems. If the intrusion persists, the grey cells concentrate in the area and release toxins that kill all cells in the area. The "immune" system can stay in this activated state for up to three weeks.<ref name="Bergquist2001PoriferaInAnderson" /> |
|||
Sponges do not have the complex [[immune system]]s of most other animals. However, they reject [[Medical grafting|grafts]] from other species but accept them from other members of their own species. In a few marine species, gray cells play the leading role in rejection of foreign material. When invaded, they produce a chemical that stops movement of other cells in the affected area, thus preventing the intruder from using the sponge's internal transport systems. If the intrusion persists, the grey cells concentrate in the area and release toxins that kill all cells in the area. The "immune" system can stay in this activated state for up to three weeks.<ref name="Bergquist_1998"/> |
|||
=== Reproduction === |
=== Reproduction === |
||
==== Asexual ==== |
==== Asexual ==== |
||
[[File:Spongilla lacustris.jpg| thumb|right|The freshwater sponge ''[[Spongilla lacustris]]'']] |
|||
Sponges have three [[asexual reproduction|asexual]] methods of reproduction: after fragmentation, by [[budding]], and by producing [[gemmule]]s. Fragments of sponges may be detached by currents or waves. They use the mobility of their [[pinacocyte]]s and [[choanocyte]]s and reshaping of the [[mesohyl]] to re-attach themselves to a suitable surface and then rebuild themselves as small but functional sponges over the course of several days. The same capabilities enable sponges that have been squeezed through a fine cloth to regenerate.{{sfn|Ruppert|Fox|Barnes|2004|p=239}} A sponge fragment can only regenerate if it contains both [[collencyte]]s to produce [[mesohyl]] and [[archeocyte]]s to produce all the other cell types.<ref name="Bergquist2001InEncOfLifeSci" /> A very few species reproduce by budding.{{sfn|Ruppert|Fox|Barnes|2004|pp=90–94}} |
|||
[[File:Spongilla lacustris.jpg|thumb|right|The freshwater sponge ''[[Spongilla lacustris]]'']] |
|||
Gemmules are "survival pods" which a few marine sponges and many freshwater species produce by the thousands when dying and which some, mainly freshwater species, regularly produce in autumn. [[Spongocyte]]s make gemmules by wrapping shells of spongin, often reinforced with spicules, round clusters of [[archeocyte]]s that are full of nutrients.{{sfn|Ruppert|Fox|Barnes|2004|pp=87–88}} Freshwater gemmules may also include photosynthesizing symbionts.<ref name="SmithPennak2001FreshwaterInvertebrates">{{cite book| vauthors = Smith DG, Pennak RW |title=Pennak's Freshwater Invertebrates of the United States: Porifera to Crustacea|publisher=[[John Wiley and Sons]]|year=2001|isbn=978-0-471-35837-4|pages=47–50| edition=4|url={{google books|plainurl=y|id=GqIctb8IqPoC|page=48}}}}</ref> The gemmules then become dormant, and in this state can survive cold, drying out, lack of oxygen and extreme variations in [[salinity]].<ref name="RuppertBarnes2004Porifera" /> Freshwater gemmules often do not revive until the temperature drops, stays cold for a few months and then reaches a near-"normal" level.<ref name="SmithPennak2001FreshwaterInvertebrates" /> When a gemmule germinates, the archeocytes round the outside of the cluster transform into [[pinacocyte]]s, a membrane over a pore in the shell bursts, the cluster of cells slowly emerges, and most of the remaining archeocytes transform into other cell types needed to make a functioning sponge. Gemmules from the same species but different individuals can join forces to form one sponge.{{sfn|Ruppert|Fox|Barnes|2004|pages=89–90}} Some gemmules are retained within the parent sponge, and in spring it can be difficult to tell whether an old sponge has revived or been "recolonized" by its own gemmules.<ref name="SmithPennak2001FreshwaterInvertebrates" /> |
|||
Sponges have three [[asexual reproduction|asexual]] methods of reproduction: after fragmentation, by [[budding]], and by producing [[gemmule]]s. Fragments of sponges may be detached by currents or waves. They use the mobility of their [[pinacocyte]]s and [[choanocyte]]s and reshaping of the [[mesohyl]] to re-attach themselves to a suitable surface and then rebuild themselves as small but functional sponges over the course of several days. The same capabilities enable sponges that have been squeezed through a fine cloth to regenerate.<ref name="Ruppert_2004"/>{{rp|239}} A sponge fragment can only regenerate if it contains both [[collencyte]]s to produce [[mesohyl]] and [[archeocyte]]s to produce all the other cell types.<ref name="Bergquist_2001"/> A very few species reproduce by budding.<ref name="Ruppert_2004"/>{{rp|90–94}} |
|||
Gemmules are "survival pods" which a few marine sponges and many freshwater species produce by the thousands when dying and which some, mainly freshwater species, regularly produce in autumn. [[Spongocyte]]s make gemmules by wrapping shells of spongin, often reinforced with spicules, round clusters of [[archeocyte]]s that are full of nutrients.<ref name="Ruppert_2004"/>{{rp|87–88}} Freshwater gemmules may also include photosynthesizing symbionts.<ref name="Smith_2001">{{cite book|author1=Smith, D. G. |author2=Pennak, R. W. |title=Pennak's Freshwater Invertebrates of the United States: Porifera to Crustacea |publisher=[[John Wiley and Sons]]|year=2001 |isbn=978-0-471-35837-4 |pages=47–50 |edition=4 |url={{google books|plainurl=y|id=GqIctb8IqPoC|page=48}}}}</ref> The gemmules then become dormant, and in this state can survive cold, drying out, lack of oxygen and extreme variations in [[salinity]].<ref name="Ruppert_2004"/> Freshwater gemmules often do not revive until the temperature drops, stays cold for a few months and then reaches a near-"normal" level.<ref name="Smith_2001"/> When a gemmule germinates, the archeocytes round the outside of the cluster transform into [[pinacocyte]]s, a membrane over a pore in the shell bursts, the cluster of cells slowly emerges, and most of the remaining archeocytes transform into other cell types needed to make a functioning sponge. Gemmules from the same species but different individuals can join forces to form one sponge.<ref name="Ruppert_2004"/>{{rp|89–90}} Some gemmules are retained within the parent sponge, and in spring it can be difficult to tell whether an old sponge has revived or been "recolonized" by its own gemmules.<ref name="Smith_2001"/> |
|||
==== Sexual ==== |
==== Sexual ==== |
||
Most sponges are [[hermaphrodite]]s (function as both sexes simultaneously), although sponges have no [[gonad]]s (reproductive organs). Sperm are produced by [[choanocyte]]s or entire choanocyte chambers that sink into the [[mesohyl]] and form spermatic [[cyst]]s while eggs are formed by transformation of [[archeocyte]]s, or of choanocytes in some species. Each egg generally acquires a [[yolk]] by consuming "nurse cells". During spawning, sperm burst out of their cysts and are expelled via the [[osculum]]. If they contact another sponge of the same species, the water flow carries them to choanocytes that engulf them but, instead of digesting them, metamorphose to an [[ameboid]] form and carry the sperm through the mesohyl to eggs, which in most cases engulf the carrier and its cargo.{{sfn|Ruppert|Fox|Barnes|2004|p=77}} |
|||
Most sponges are [[hermaphrodite]]s (function as both sexes simultaneously), although sponges have no [[gonad]]s (reproductive organs). Sperm are produced by [[choanocyte]]s or entire choanocyte chambers that sink into the [[mesohyl]] and form spermatic [[cyst]]s while eggs are formed by transformation of [[archeocyte]]s, or of choanocytes in some species. Each egg generally acquires a [[yolk]] by consuming "nurse cells". During spawning, sperm burst out of their cysts and are expelled via the [[osculum]]. If they contact another sponge of the same species, the water flow carries them to choanocytes that engulf them but, instead of digesting them, metamorphose to an [[ameboid]] form and carry the sperm through the mesohyl to eggs, which in most cases engulf the carrier and its cargo.<ref name="Ruppert_2004"/>{{rp|77}} |
|||
A few species release fertilized eggs into the water, but most retain the eggs until they hatch. There are four types of larvae, but all are balls of cells with an outer layer of cells whose [[flagella]]e or [[cilia]] enable the larvae to move. After swimming for a few days the larvae sink and crawl until they find a place to settle. Most of the cells transform into archeocytes and then into the types appropriate for their locations in a miniature adult sponge.{{sfn|Ruppert|Fox|Barnes|2004|p=77}} |
|||
A few species release fertilized eggs into the water, but most retain the eggs until they hatch. By retaining the eggs, the parents can transfer symbiotic microorganisms directly to their offspring through [[vertical transmission]], while the species who release their eggs into the water has to acquire symbionts horizontally (a combination of both is probably most common, where larvae with vertically transmitted symbionts also acquire others horizontally).<ref>{{cite journal |last1=Díez-Vives |first1=Cristina |last2=Koutsouveli |first2=Vasiliki |last3=Conejero |first3=Maria |last4=Riesgo |first4=Ana |title=Global patterns in symbiont selection and transmission strategies in sponges |journal=Frontiers in Ecology and Evolution |volume=10 |date=26 October 2022 |issn=2296-701X |doi=10.3389/fevo.2022.1015592 |doi-access=free}}</ref><ref>{{cite journal |last1=Carrier |first1=Tyler J. |last2=Maldonado |first2=Manuel |last3=Schmittmann |first3=Lara |last4=Pita |first4=Lucía |last5=Bosch |first5=Thomas C. G. |last6=Hentschel |first6=Ute |title=Symbiont transmission in marine sponges: reproduction, development, and metamorphosis |journal=BMC Biology |volume=20 |issue=1 |pages=100 |date=May 2022 |pmid=35524305 |pmc=9077847 |doi=10.1186/s12915-022-01291-6 |doi-access=free }}</ref> There are four types of larvae, but all are lecithotrophic (non-feeding) balls of cells with an outer layer of cells whose [[flagella]] or [[cilia]] enable the larvae to move. After swimming for a few days the larvae sink and crawl until they find a place to settle. Most of the cells transform into archeocytes and then into the types appropriate for their locations in a miniature adult sponge.<ref name="Ruppert_2004"/>{{rp|77}}<ref>{{cite journal |last1=Riesgo |first1=Ana |last2=Taboada |first2=Sergio |last3=Sánchez-Vila |first3=Laura |last4=Solà |first4=Joan |last5=Bertran |first5=Andrea |last6=Avila |first6=Conxita |title=Some Like It Fat: Comparative Ultrastructure of the Embryo in Two Demosponges of the Genus Mycale (Order Poecilosclerida) from Antarctica and the Caribbean |journal=PLOS ONE |volume=10 |issue=3 |date=18 March 2015 |issn=1932-6203 |pmid=25785444 |pmc=4365022 |doi=10.1371/journal.pone.0118805 |doi-access=free |page=e0118805|bibcode=2015PLoSO..1018805R }}</ref> |
|||
[[Glass sponge]] embryos start by dividing into separate cells, but once 32 cells have formed they rapidly transform into larvae that externally are [[ovoid]] with a band of [[cilia]] round the middle that they use for movement, but internally have the typical glass sponge structure of spicules with a cobweb-like main [[syncitium]] draped around and between them and [[choanosyncytia]] with multiple collar bodies in the center. The larvae then leave their parents' bodies.<ref>{{cite journal | vauthors = Leys SP, Cheung E, Boury-Esnault N | title = Embryogenesis in the glass sponge Oopsacas minuta: Formation of syncytia by fusion of blastomeres | journal = Integrative and Comparative Biology | volume = 46 | issue = 2 | pages = 104–17 | date = April 2006 | pmid = 21672727 | doi = 10.1093/icb/icj016 | doi-access = free }}</ref> |
|||
[[Glass sponge]] embryos start by dividing into separate cells, but once 32 cells have formed they rapidly transform into larvae that externally are [[ovoid]] with a band of [[cilia]] round the middle that they use for movement, but internally have the typical glass sponge structure of spicules with a cobweb-like main [[syncitium]] draped around and between them and [[choanosyncytia]] with multiple collar bodies in the center. The larvae then leave their parents' bodies.<ref>{{cite journal |last=Leys |first=S. P. |title=Embryogenesis in the glass sponge Oopsacas minuta: Formation of syncytia by fusion of blastomeres |journal=Integrative and Comparative Biology |volume=46 |issue=2 |date=16 February 2006 |issn=1540-7063 |doi=10.1093/icb/icj016 |pages=104–117|pmid=21672727 }}</ref> |
|||
===Meiosis=== |
|||
The cytological progression of porifera [[oogenesis]] and [[spermatogenesis]] ([[gametogenesis]]) is very similar to that of other metazoa.<ref name="Koutsouveli_2020">{{cite journal |last1=Koutsouveli |first1=Vasiliki |last2=Cárdenas |first2=Paco |last3=Santodomingo |first3=Nadiezhda |last4=Marina |first4=Anabel |last5=Morato |first5=Esperanza |last6=Rapp |first6=Hans Tore |last7=Riesgo |first7=Ana |title=The Molecular Machinery of Gametogenesis in Geodia Demosponges (Porifera): Evolutionary Origins of a Conserved Toolkit across Animals |journal=Molecular Biology and Evolution |volume=37 |issue=12 |date=16 December 2020 |issn=0737-4038 |pmid=32929503 |pmc=7743902 |doi=10.1093/molbev/msaa183 |pages=3485–3506}}</ref> Most of the genes from the classic set of [[meiosis|meiotic]] genes, including genes for DNA recombination and double-strand break repair, that are conserved in [[eukaryote]]s are expressed in the sponges (e.g. ''[[Geodia hentscheli]]'' and ''Geodia phlegraei'').<ref name="Koutsouveli_2020"/> Since porifera are considered to be the earliest divergent animals, these findings indicate that the basic toolkit of meiosis including capabilities for recombination and DNA repair were present early in eukaryote evolution.<ref name="Koutsouveli_2020"/> |
|||
==== Life cycle ==== |
==== Life cycle ==== |
||
Sponges in [[temperate]] regions live for at most a few years, but some [[Tropics|tropical]] species and perhaps some deep-ocean ones may live for 200 years or more. Some calcified [[demosponge]]s grow by only {{convert|0.2|mm|in|abbr=on}} per year and, if that rate is constant, specimens {{convert|1|m|ft|abbr=on}} wide must be about 5,000 years old. Some sponges start sexual reproduction when only a few weeks old, while others wait until they are several years old.<ref name="RuppertBarnes2004Porifera" /> |
|||
Sponges in [[temperate]] regions live for at most a few years, but some [[Tropics|tropical]] species and perhaps some deep-ocean ones may live for 200 years or more. Some calcified [[demosponge]]s grow by only {{convert|0.2|mm|in|abbr=on}} per year and, if that rate is constant, specimens {{convert|1|m|ft|abbr=on}} wide must be about 5,000 years old. Some sponges start sexual reproduction when only a few weeks old, while others wait until they are several years old.<ref name="Ruppert_2004"/> |
|||
=== Coordination of activities === |
=== Coordination of activities === |
||
Adult sponges lack [[neuron]]s or any other kind of [[nervous tissue]]. However, most species have the ability to perform movements that are coordinated all over their bodies, mainly contractions of the [[pinacocyte]]s, squeezing the water channels and thus expelling excess sediment and other substances that may cause blockages. Some species can contract the [[osculum]] independently of the rest of the body. Sponges may also contract in order to reduce the area that is vulnerable to attack by predators. In cases where two sponges are fused, for example if there is a large but still unseparated bud, these contraction waves slowly become coordinated in both of the "[[Siamese twins]]". The coordinating mechanism is unknown, but may involve chemicals similar to [[neurotransmitter]]s.<ref>{{cite journal | vauthors = Nickel M | title = Kinetics and rhythm of body contractions in the sponge Tethya wilhelma (Porifera: Demospongiae) | journal = The Journal of Experimental Biology | volume = 207 | issue = Pt 26 | pages = 4515–24 | date = December 2004 | pmid = 15579547 | doi = 10.1242/jeb.01289 | doi-access = free }}</ref> However, [[glass sponge]]s rapidly transmit electrical impulses through all parts of the [[syncytium]], and use this to halt the motion of their [[flagella]] if the incoming water contains toxins or excessive sediment.<ref name="RuppertBarnes2004Porifera" /> [[Myocyte]]s are thought to be responsible for closing the osculum and for transmitting signals between different parts of the body.<ref name="Bergquist2001PoriferaInAnderson" /> |
|||
Adult sponges lack [[neuron]]s or any other kind of [[nervous tissue]]. However, most species have the ability to perform movements that are coordinated all over their bodies, mainly contractions of the [[pinacocyte]]s, squeezing the water channels and thus expelling excess sediment and other substances that may cause blockages. Some species can contract the [[osculum]] independently of the rest of the body. Sponges may also contract in order to reduce the area that is vulnerable to attack by predators. In cases where two sponges are fused, for example if there is a large but still unseparated bud, these contraction waves slowly become coordinated in both of the "[[Siamese twins]]". The coordinating mechanism is unknown, but may involve chemicals similar to [[neurotransmitter]]s.<ref>{{cite journal |last=Nickel |first=M. |title=Kinetics and rhythm of body contractions in the sponge Tethya wilhelma (Porifera: Demospongiae) |journal=The Journal of Experimental Biology |volume=207 |issue=Pt 26 |pages=4515–24 |date=December 2004 |pmid=15579547 |doi=10.1242/jeb.01289 |doi-access=free }}</ref> However, [[glass sponge]]s rapidly transmit electrical impulses through all parts of the [[syncytium]], and use this to halt the motion of their [[flagella]] if the incoming water contains toxins or excessive sediment.<ref name="Ruppert_2004"/> [[Myocyte]]s are thought to be responsible for closing the osculum and for transmitting signals between different parts of the body.<ref name="Bergquist_1998"/> |
|||
Sponges contain [[gene]]s very similar to those that contain the "recipe" for the post-[[synapse|synaptic]] density, an important signal-receiving structure in the neurons of all other animals. However, in sponges these genes are only activated in "flask cells" that appear only in larvae and may provide some sensory capability while the larvae are swimming. This raises questions about whether flask cells represent the predecessors of true neurons or are evidence that sponges' ancestors had true neurons but lost them as they adapted to a sessile lifestyle.<ref>{{cite journal | vauthors = Sakarya O, Armstrong KA, Adamska M, Adamski M, Wang IF, Tidor B, Degnan BM, Oakley TH, Kosik KS | display-authors = 6 | title = A post-synaptic scaffold at the origin of the animal kingdom | journal = PLOS ONE | volume = 2 | issue = 6 | pages = e506 | date = June 2007 | pmid = 17551586 | pmc = 1876816 | doi = 10.1371/journal.pone.0000506 | bibcode = 2007PLoSO...2..506S | doi-access = free }}</ref> |
|||
Sponges contain [[gene]]s very similar to those that contain the "recipe" for the post-[[synapse|synaptic]] density, an important signal-receiving structure in the neurons of all other animals. However, in sponges these genes are only activated in "flask cells" that appear only in larvae and may provide some sensory capability while the larvae are swimming. This raises questions about whether flask cells represent the predecessors of true neurons or are evidence that sponges' ancestors had true neurons but lost them as they adapted to a sessile lifestyle.<ref>{{cite journal |last1=Sakarya |first1=Onur |last2=Armstrong |first2=Kathryn A. |last3=Adamska |first3=Maja |last4=Adamski |first4=Marcin |last5=Wang |first5=I-Fan |last6=Tidor |first6=Bruce |last7=Degnan |first7=Bernard M. |last8=Oakley |first8=Todd H. |last9=Kosik |first9=Kenneth S. |title=A Post-Synaptic Scaffold at the Origin of the Animal Kingdom |journal=PLOS ONE |volume=2 |issue=6 |date=6 June 2007 |issn=1932-6203 |pmid=17551586 |pmc=1876816 |doi=10.1371/journal.pone.0000506 |doi-access=free |page=e506|bibcode=2007PLoSO...2..506S }}</ref> |
|||
<!-- |
<!-- |
||
Sponges have several cell types: |
Sponges have several cell types: |
||
| Line 240: | Line 222: | ||
* [[Myocytes (sponge)|Myocytes]] are modified pinacocytes which control the size of the osculum and pore openings and thus the water flow. |
* [[Myocytes (sponge)|Myocytes]] are modified pinacocytes which control the size of the osculum and pore openings and thus the water flow. |
||
* [[Pinacocytes]] which form the [[pinacoderm]], the outer epidermal layer of cells. This is the closest approach to true tissue in sponges |
* [[Pinacocytes]] which form the [[pinacoderm]], the outer epidermal layer of cells. This is the closest approach to true tissue in sponges |
||
* [[Porocytes]] are tubular cells that make up |
* [[Porocytes]] are tubular cells that make up the sponge's pores. |
||
* [[Sclerocytes]] secrete [[calcareous]] siliceous spicules which reside in the mesohyl. |
* [[Sclerocytes]] secrete [[calcareous]] siliceous spicules which reside in the mesohyl. |
||
* [[Spongocytes]] secrete [[spongin]], [[collagen]]-like fibers which make up the mesohyl. |
* [[Spongocytes]] secrete [[spongin]], [[collagen]]-like fibers which make up the mesohyl. |
||
| Line 249: | Line 231: | ||
=== Habitats === |
=== Habitats === |
||
{{See also|Sponge ground|Sponge reef}} |
{{See also|Sponge ground|Sponge reef}} |
||
[[File:Euplectella aspergillum (cropped).jpg|thumb|upright=1.2|''[[Euplectella aspergillum]]'' is a [[deep ocean]] [[glass sponge]]; seen here at a depth of {{convert|2572|m}} off the coast of [[California]]]] |
[[File:Euplectella aspergillum (cropped).jpg|thumb|upright=1.2|''[[Euplectella aspergillum]]'' is a [[deep ocean]] [[glass sponge]]; seen here at a depth of {{convert|2572|m}} off the coast of [[California]]]] |
||
Sponges are worldwide in their distribution, living in a wide range of ocean habitats, from the polar regions to the tropics.<ref name=" |
Sponges are worldwide in their distribution, living in a wide range of ocean habitats, from the polar regions to the tropics.<ref name="Bergquist_2001"/> Most live in quiet, clear waters, because sediment stirred up by waves or currents would block their pores, making it difficult for them to feed and breathe.<ref name="Krautter_1998"/> The greatest numbers of sponges are usually found on firm surfaces such as rocks, but some sponges can attach themselves to soft sediment by means of a root-like base.<ref>{{cite journal |last1=Weaver |first1=James C. |last2=Aizenberg |first2=Joanna |last3=Fantner |first3=Georg E. |last4=Kisailus |first4=David |last5=Woesz |first5=Alexander |last6=Allen |first6=Peter |last7=Fields |first7=Kirk |last8=Porter |first8=Michael J. |last9=Zok |first9=Frank W. |last10=Hansma |first10=Paul K. |last11=Fratzl |first11=Peter |last12=Morse |first12=Daniel E. |title=Hierarchical assembly of the siliceous skeletal lattice of the hexactinellid sponge Euplectella aspergillum |journal=Journal of Structural Biology |volume=158 |issue=1 |date=2007 |doi=10.1016/j.jsb.2006.10.027 |pages=93–106|pmid=17175169 }}</ref> |
||
Sponges are more abundant but less diverse in temperate waters than in tropical waters, possibly because organisms that prey on sponges are more abundant in tropical waters.<ref>{{cite journal | |
Sponges are more abundant but less diverse in temperate waters than in tropical waters, possibly because organisms that prey on sponges are more abundant in tropical waters.<ref>{{cite journal |last1= Ruzicka |first1=R |last2=Gleason |first2=D.F. |title=Latitudinal variation in spongivorous fishes and the effectiveness of sponge chemical defenses |journal=Oecologia |volume=154 |issue=4 |pages=785–94 |date=January 2008 |pmid=17960425 |doi=10.1007/s00442-007-0874-0 |url=http://www.bio.georgiasouthern.edu/Bio-home/Gleason/Ruzicka&Gleasonfulltext.pdf |archive-url=https://web.archive.org/web/20081006123601/http://www.bio.georgiasouthern.edu/Bio-home/Gleason/Ruzicka%26Gleasonfulltext.pdf |url-status=dead |bibcode=2008Oecol.154..785R |s2cid=1495896 |archive-date=2008-10-06 }}</ref> [[Glass sponge]]s are the most common in polar waters and in the depths of temperate and tropical seas, as their very porous construction enables them to extract food from these resource-poor waters with the minimum of effort. [[Demosponge]]s and [[Calcarea|calcareous sponges]] are abundant and diverse in shallower non-polar waters.<ref>{{cite book |last1=Gage |first1=J.D. |last2=Tyler |first2=P.A. |title=Deep-sea Biology: A Natural History of Organisms at the Deep-Sea Floor|publisher=[[Cambridge University Press]] |pages=91–93 |year=1996|isbn=978-0-521-33665-9|url={{google books |plainurl=y |id=kHZO5igKhsAC}}}}</ref> |
||
The different [[Class (biology)|classes]] of sponge live in different ranges of habitat: |
The different [[Class (biology)|classes]] of sponge live in different ranges of habitat: |
||
{{Clear}} |
{{Clear}} |
||
:{| |
:{|class="wikitable" |
||
|- |
|- |
||
! [[Class (biology)|Class]] !! Water type<ref name=" |
! [[Class (biology)|Class]] !! Water type<ref name="Bergquist_1998"/> !! Depth<ref name="Bergquist_1998"/> !! Type of surface<ref name="Bergquist_1998"/> |
||
|- |
|- |
||
! [[Calcarea]] |
! [[Calcarea]] |
||
| |
|Marine ||less than {{convert|100|m|ft|abbr=on}} ||Hard |
||
|- |
|- |
||
! [[Glass sponge]]s |
! [[Glass sponge]]s |
||
| |
|Marine ||Deep ||Soft or firm [[sediment]] |
||
|- |
|- |
||
! [[Demosponge]]s |
! [[Demosponge]]s |
||
| |
|Marine, brackish; and about 150 freshwater species<ref name="Ruppert_2004"/> ||Inter-tidal to abyssal;<ref name="Bergquist_1998"/> a carnivorous demosponge has been found at {{convert|8840|m|mi|abbr=on}}<ref name="Vacelet_1995"/> ||Any |
||
|} |
|} |
||
=== As primary producers === |
=== As primary producers === |
||
Sponges with [[photosynthesis|photosynthesizing]] [[endosymbiont]]s produce up to three times more [[oxygen]] than they consume, as well as more organic matter than they consume. Such contributions to their habitats' resources are significant along Australia's [[Great Barrier Reef]] but relatively minor in the Caribbean.<ref name="Bergquist2001InEncOfLifeSci" /> |
|||
Sponges with [[photosynthesis|photosynthesizing]] [[endosymbiont]]s produce up to three times more [[oxygen]] than they consume, as well as more organic matter than they consume. Such contributions to their habitats' resources are significant along Australia's [[Great Barrier Reef]] but relatively minor in the Caribbean.<ref name="Bergquist_2001"/> |
|||
=== Defenses === |
=== Defenses === |
||
[[File:BoredEncrustedShell.JPG|thumb|right|Holes made by clionaid sponge (producing the trace ''[[Entobia]]'') after the death of a modern bivalve shell of species ''[[Mercenaria mercenaria]]'', from [[North Carolina]]]] |
[[File:BoredEncrustedShell.JPG|thumb|right|Holes made by clionaid sponge (producing the trace ''[[Entobia]]'') after the death of a modern bivalve shell of species ''[[Mercenaria mercenaria]]'', from [[North Carolina]]]] |
||
[[File:Entobia Modern.jpg|thumb|Close-up of the sponge boring ''Entobia'' in a modern oyster valve. Note the chambers |
[[File:Entobia Modern.jpg|thumb|Close-up of the sponge boring ''Entobia'' in a modern oyster valve. Note the chambers connected by short tunnels.]] |
||
Many sponges shed [[Sponge spicule|spicules]], forming a dense carpet several meters deep that keeps away [[echinoderm]]s which would otherwise prey on the sponges.<ref name="Bergquist2001InEncOfLifeSci" /> They also produce toxins that prevent other sessile organisms such as [[bryozoa]]ns or [[sea squirt]]s from growing on or near them, making sponges very effective competitors for living space. One of many examples includes [[ageliferin]]. |
|||
Many sponges shed [[Sponge spicule|spicules]], forming a dense carpet several meters deep that keeps away [[echinoderm]]s which would otherwise prey on the sponges.<ref name="Bergquist_2001"/> They also produce toxins that prevent other sessile organisms such as [[bryozoa]]ns or [[sea squirt]]s from growing on or near them, making sponges very effective competitors for living space. One of many examples includes [[ageliferin]]. |
|||
A few species, the [[Caribbean]] fire sponge ''[[Tedania]] ignis'', cause a severe rash in humans who handle them.<ref name="RuppertBarnes2004Porifera" /> Turtles and some fish feed mainly on sponges. It is often said that sponges produce [[chemical defense]]s against such predators.<ref name="RuppertBarnes2004Porifera" /> However, experiments have been unable to establish a relationship between the toxicity of chemicals produced by sponges and how they taste to fish, which would diminish the usefulness of chemical defenses as deterrents. Predation by fish may even help to spread sponges by detaching fragments.<ref name="Bergquist2001PoriferaInAnderson" /> However, some studies have shown fish showing a preference for non chemically defended sponges,<ref>{{Cite journal| vauthors = Dunlap M, Pawlik JR |date=1996|title=Video-monitored predation by Caribbean reef fishes on an array of mangrove and reef sponges|journal=Marine Biology|volume=126|issue=1|pages=117–123|doi=10.1007/bf00571383|s2cid=84799900|issn=0025-3162}}</ref> and another study found that high levels of coral predation did predict the presence of chemically defended species.<ref>{{cite journal | vauthors = Loh TL, Pawlik JR | title = Chemical defenses and resource trade-offs structure sponge communities on Caribbean coral reefs | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 111 | issue = 11 | pages = 4151–6 | date = March 2014 | pmid = 24567392 | pmc = 3964098 | doi = 10.1073/pnas.1321626111 | bibcode = 2014PNAS..111.4151L | doi-access = free }}</ref> |
|||
A few species, the [[Caribbean]] fire sponge ''[[Tedania]] ignis'', cause a severe rash in humans who handle them.<ref name="Ruppert_2004"/> Turtles and some fish feed mainly on sponges. It is often said that sponges produce [[chemical defense]]s against such predators.<ref name="Ruppert_2004"/> However, experiments have been unable to establish a relationship between the toxicity of chemicals produced by sponges and how they taste to fish, which would diminish the usefulness of chemical defenses as deterrents. Predation by fish may even help to spread sponges by detaching fragments.<ref name="Bergquist_1998"/> However, some studies have shown fish showing a preference for non chemically defended sponges,<ref>{{Cite journal |last1=Dunlap |first1=M. |last2=Pawlik |first2=J. R. |date=1996|title=Video-monitored predation by Caribbean reef fishes on an array of mangrove and reef sponges|journal=Marine Biology|volume=126|issue=1 |pages=117–123|doi=10.1007/bf00571383 |bibcode=1996MarBi.126..117D |s2cid=84799900|issn=0025-3162}}</ref> and another study found that high levels of coral predation did predict the presence of chemically defended species.<ref>{{cite journal |last1=Loh |first1=T. L. |last2=Pawlik |first2=J. R. |title=Chemical defenses and resource trade-offs structure sponge communities on Caribbean coral reefs |journal=Proceedings of the National Academy of Sciences of the United States of America |volume=111 |issue=11 |pages=4151–6 |date=March 2014 |pmid=24567392 |pmc=3964098 |doi=10.1073/pnas.1321626111 |bibcode=2014PNAS..111.4151L |doi-access=free }}</ref> |
|||
[[Glass sponge]]s produce no toxic chemicals, and live in very deep water where predators are rare.<ref name="Krautter1998EcologyOfSiliceousSponges" /> |
|||
[[Glass sponge]]s produce no toxic chemicals, and live in very deep water where predators are rare.<ref name="Krautter_1998"/> |
|||
=== Predation === |
=== Predation === |
||
{{See also|Spongivore}} |
|||
{{further|Spongivore}} |
|||
Spongeflies, also known as spongillaflies ([[Neuroptera]], [[Sisyridae]]), are specialist predators of freshwater sponges. The female lays her eggs on vegetation overhanging water. The larvae hatch and drop into the water where they seek out sponges to feed on. They use their elongated mouthparts to pierce the sponge and suck the fluids within. The larvae of some species cling to the surface of the sponge while others take refuge in the sponge's internal cavities. The fully grown larvae leave the water and spin a cocoon in which to pupate.{{sfn|Piper|2007|p=148}} |
|||
Spongeflies, also known as spongillaflies ([[Neuroptera]], [[Sisyridae]]), are specialist predators of freshwater sponges. The female lays her eggs on vegetation overhanging water. The larvae hatch and drop into the water where they seek out sponges to feed on. They use their elongated mouthparts to pierce the sponge and suck the fluids within. The larvae of some species cling to the surface of the sponge while others take refuge in the sponge's internal cavities. The fully grown larvae leave the water and spin a cocoon in which to pupate.<ref>{{cite book |last=Piper |first=Ross |author-link1=Ross Piper |year=2007 |title=Extraordinary Animals: An Encyclopedia of Curious and Unusual Animals |publisher=[[Greenwood Publishing Group]] |isbn=978-0-313-33922-6 |url-access=registration |url=https://archive.org/details/extraordinaryani0000pipe |page=148}}</ref> |
|||
=== Bioerosion === |
=== Bioerosion === |
||
The Caribbean chicken-liver sponge ''[[Chondrilla nucula]]'' secretes toxins that kill coral [[polyp (zoology)|polyps]], allowing the sponges to grow over the coral skeletons.<ref name="RuppertBarnes2004Porifera" /> Others, especially in the family [[Clionaidae]], use corrosive substances secreted by their archeocytes to tunnel into rocks, corals and the shells of dead [[mollusk]]s.<ref name="RuppertBarnes2004Porifera" /> Sponges may remove up to {{convert|1|m|ft|abbr=on}} per year from reefs, creating visible notches just below low-tide level.<ref name="Bergquist2001InEncOfLifeSci" /> |
|||
The Caribbean chicken-liver sponge ''[[Chondrilla nucula]]'' secretes toxins that kill coral [[polyp (zoology)|polyps]], allowing the sponges to grow over the coral skeletons.<ref name="Ruppert_2004"/> Others, especially in the family [[Clionaidae]], use corrosive substances secreted by their archeocytes to tunnel into rocks, corals and the shells of dead [[mollusk]]s.<ref name="Ruppert_2004"/> Sponges may remove up to {{convert|1|m|ft|abbr=on}} per year from reefs, creating visible notches just below low-tide level.<ref name="Bergquist_2001"/> |
|||
=== Diseases === |
=== Diseases === |
||
Caribbean sponges of the genus ''[[Aplysina]]'' suffer from [[Aplysina red band syndrome]]. This causes ''Aplysina'' to develop one or more rust-colored bands, sometimes with adjacent bands of [[necrosis|necrotic]] tissue. These lesions may completely encircle branches of the sponge. The disease appears to be [[Contagious disease|contagious]] and impacts approximately 10 percent of ''A. cauliformis'' on Bahamian reefs.<ref name=Gochfeld2012>{{cite journal|vauthors=Gochfeld DJ, Easson CG, Slattery M, Thacker RW, Olson JB|title=Population Dynamics of a Sponge Disease on Caribbean Reefs|journal=Diving for Science 2012|veditors=Steller D, Lobel L|publisher=Proceedings of the American Academy of Underwater Sciences 31st Symposium|year=2012|url=http://archive.rubicon-foundation.org/10327|access-date=2013-11-17|archive-url=https://web.archive.org/web/20150904045439/http://archive.rubicon-foundation.org/xmlui/handle/123456789/10327|archive-date=2015-09-04|url-status=usurped}}</ref> The rust-colored bands are caused by a [[cyanobacterium]], but it is unknown whether this organism actually causes the disease.<ref name=Gochfeld2012 /><ref>{{cite journal | vauthors = Olson JB, Gochfeld DJ, Slattery M | title = Aplysina red band syndrome: a new threat to Caribbean sponges | journal = Diseases of Aquatic Organisms | volume = 71 | issue = 2 | pages = 163–8 | date = July 2006 | pmid = 16956064 | doi = 10.3354/dao071163 | doi-access = free }} |
|||
Caribbean sponges of the genus ''[[Aplysina]]'' suffer from [[Aplysina red band syndrome]]. This causes ''Aplysina'' to develop one or more rust-colored bands, sometimes with adjacent bands of [[necrosis|necrotic]] tissue. These lesions may completely encircle branches of the sponge. The disease appears to be [[Contagious disease|contagious]] and impacts approximately 10 percent of ''A. cauliformis'' on Bahamian reefs.<ref name="Gochfeld_2012">{{cite journal|last=Gochfeld |first=D.J. |display-authors=etal |title=Population Dynamics of a Sponge Disease on Caribbean Reefs |journal=Diving for Science 2012 |editor1=Steller, D. |editor2=Lobel, L. |publisher=Proceedings of the American Academy of Underwater Sciences 31st Symposium |year=2012|url=http://archive.rubicon-foundation.org/10327 |archive-url=https://web.archive.org/web/20150904045439/http://archive.rubicon-foundation.org/xmlui/handle/123456789/10327 |archive-date=2015-09-04 |url-status=usurped}}</ref> The rust-colored bands are caused by a [[cyanobacterium]], but it is unknown whether this organism actually causes the disease.<ref name="Gochfeld_2012"/><ref>{{cite journal |last1=Olson |first1=J.B. |last2=Gochfeld |first2=D.J. |last3=Slattery |first3=M. |title=Aplysina red band syndrome: a new threat to Caribbean sponges |journal=Diseases of Aquatic Organisms |volume=71 |issue=2 |pages=163–8 |date=July 2006 |pmid=16956064 |doi=10.3354/dao071163 |doi-access=free }} {{lay source |template=cite web |last=Clarke |first=M. |date=2006-10-17 |title=New disease threatens sponges |website=Practical Fishkeeping |url=http://www.practicalfishkeeping.co.uk/pfk/pages/item.php?news=1113 |archive-url=https://web.archive.org/web/20070926235654/http://www.practicalfishkeeping.co.uk/pfk/pages/item.php?news=1113 |archive-date=2007-09-26}}</ref> |
|||
*{{cite web |author=Matt Clarke |date=2006-10-17 |title=New disease threatens sponges |website=Practical Fishkeeping |url=http://www.practicalfishkeeping.co.uk/pfk/pages/item.php?news=1113 |archive-url=https://web.archive.org/web/20070926235654/http://www.practicalfishkeeping.co.uk/pfk/pages/item.php?news=1113 |archive-date=2007-09-26}}</ref> |
|||
=== Collaboration with other organisms === |
=== Collaboration with other organisms === |
||
In addition to hosting photosynthesizing endosymbionts,<ref name="RuppertBarnes2004Porifera" /> sponges are noted for their wide range of collaborations with other organisms. The relatively large encrusting sponge ''[[Lissodendoryx]] colombiensis'' is most common on rocky surfaces, but has extended its range into [[seagrass meadow]]s by letting itself be surrounded or overgrown by seagrass sponges, which are distasteful to the local [[starfish]] and therefore protect ''Lissodendoryx'' against them; in return, the seagrass sponges get higher positions away from the sea-floor sediment.<ref>{{cite journal| vauthors = Wulff JL |title=Collaboration among sponge species increases sponge diversity and abundance in a seagrass meadow|journal=[[Marine Ecology Progress Series|Marine Ecology]]|volume=29|issue=2|date=June 2008 |pages=193–204|doi=10.1111/j.1439-0485.2008.00224.x|bibcode=2008MarEc..29..193W|doi-access=free}}</ref> |
|||
In addition to hosting photosynthesizing endosymbionts,<ref name="Ruppert_2004"/> sponges are noted for their wide range of collaborations with other organisms. The relatively large encrusting sponge ''[[Lissodendoryx]] colombiensis'' is most common on rocky surfaces, but has extended its range into [[seagrass meadow]]s by letting itself be surrounded or overgrown by seagrass sponges, which are distasteful to the local [[starfish]] and therefore protect ''Lissodendoryx'' against them; in return, the seagrass sponges get higher positions away from the sea-floor sediment.<ref>{{cite journal|last=Wulff |first=J. L. |title=Collaboration among sponge species increases sponge diversity and abundance in a seagrass meadow|journal=[[Marine Ecology Progress Series|Marine Ecology]] |volume=29|issue=2|date=June 2008 |pages=193–204 |doi=10.1111/j.1439-0485.2008.00224.x |bibcode=2008MarEc..29..193W |doi-access=free}}</ref> |
|||
[[Shrimp]]s of the genus ''[[Synalpheus]]'' form colonies in sponges, and each shrimp species inhabits a different sponge species, making ''Synalpheus'' one of the most diverse [[crustacea]]n genera. Specifically, [[Synalpheus regalis]] utilizes the sponge not only as a food source, but also as a defense against other shrimp and predators.<ref>{{cite journal | vauthors = Duffy JE |year=1996 |title=Species boundaries, specialization, and the radiation of sponge-dwelling alpheid shrimp |journal=[[Biological Journal of the Linnean Society]] |volume=58 |issue=3 |pages=307–324 |doi=10.1111/j.1095-8312.1996.tb01437.x |doi-access=free }}</ref> As many as 16,000 individuals inhabit a single [[loggerhead sponge]], feeding off the larger particles that collect on the sponge as it filters the ocean to feed itself.{{sfn|Murphy|2002|page=51}} Other crustaceans such as hermit crabs commonly have a specific species of sponge, ''[[Pseudospongosorites]]'', grow on them as both the sponge and crab occupy gastropod shells until the crab and sponge outgrow the shell, eventually resulting in the crab using the sponge's body as protection instead of the shell until the crab finds a suitable replacement shell.<ref>{{cite journal |title=Population dynamics and epibiont associations of hermit crabs (Crustacea: Decapoda: Paguroidea) on Dog Island, Florida |journal=Memoirs of Museum Victoria |year=2003 |last=Sandford |first=Floyd |volume=60 |issue=1 |pages=45–52 |doi=10.24199/j.mmv.2003.60.6 |s2cid=86167606 |issn=1447-2554 |url=https://museumsvictoria.com.au/media/4034/60_1_sandford.pdf |archive-url=https://web.archive.org/web/20180719145946/https://museumsvictoria.com.au/media/4034/60_1_sandford.pdf |archive-date=2018-07-19 |url-status=live |access-date=2022-01-24 }}</ref> |
|||
[[Shrimp]]s of the genus ''[[Synalpheus]]'' form colonies in sponges, and each shrimp species inhabits a different sponge species, making ''Synalpheus'' one of the most diverse [[crustacea]]n genera. Specifically, [[Synalpheus regalis]] utilizes the sponge not only as a food source, but also as a defense against other shrimp and predators.<ref>{{cite journal |last= Duffy |first=J. E. |year=1996 |title=Species boundaries, specialization, and the radiation of sponge-dwelling alpheid shrimp |journal=[[Biological Journal of the Linnean Society]] |volume=58 |issue=3 |pages=307–324 |doi=10.1111/j.1095-8312.1996.tb01437.x |doi-access=free }}</ref> As many as 16,000 individuals inhabit a single [[loggerhead sponge]], feeding off the larger particles that collect on the sponge as it filters the ocean to feed itself.<ref>{{cite book |last= Murphy |first=R.C. |title= Coral Reefs: Cities Under The Seas |year=2002|isbn=978-0-87850-138-0|publisher=[[The Darwin Press]], Inc. |page=51}}</ref> Other crustaceans such as hermit crabs commonly have a specific species of sponge, ''[[Pseudospongosorites]]'', grow on them as both the sponge and crab occupy gastropod shells until the crab and sponge outgrow the shell, eventually resulting in the crab using the sponge's body as protection instead of the shell until the crab finds a suitable replacement shell.<ref>{{cite journal |last=Sandford |first=F. |title=Population dynamics and epibiont associations of hermit crabs (Crustacea: Decapoda: Paguroidea) on Dog Island, Florida |journal=Memoirs of Museum Victoria |year=2003|volume=60 |issue=1 |pages=45–52 |doi=10.24199/j.mmv.2003.60.6 |s2cid=86167606 |issn=1447-2554 |url=https://museumsvictoria.com.au/media/4034/60_1_sandford.pdf |archive-url=https://web.archive.org/web/20180719145946/https://museumsvictoria.com.au/media/4034/60_1_sandford.pdf |archive-date=2018-07-19 |url-status=live |access-date=2022-01-24 }}</ref> |
|||
[[File:Bathymetrical range of selected sponge species.jpg|thumb|upright=1.5|left|Bathymetrical range of some sponge species.<ref>{{cite journal | last=Łukowiak | first=Magdalena | title=Utilizing sponge spicules in taxonomic, ecological and environmental reconstructions: a review | journal=PeerJ | volume=8 | date=18 December 2020 | issn=2167-8359 | doi=10.7717/peerj.10601 | page=e10601| pmid=33384908 | pmc=7751429 | doi-access=free }}</ref> Demosponge ''[[Samus anonymus]]'' (up to 50 m), [[hexactinellid]] ''Scleroplegma lanterna'' (~100–600 m), hexactinellid ''Aulocalyx irregularis'' (~550–915 m), lithistid demosponge ''Neoaulaxinia persicum'' (~500–1700 m)]] |
|||
[[File:Bathymetrical range of selected sponge species.jpg|thumb|upright=1.5|left|Bathymetrical range of some sponge species.<ref>{{cite journal |last=Łukowiak |first=M. |title=Utilizing sponge spicules in taxonomic, ecological and environmental reconstructions: a review |journal=PeerJ |volume=8 |pages=e10601 |date=18 December 2020 |pmid=33384908 |pmc=7751429 |doi=10.7717/peerj.10601 |doi-access=free }}</ref> Demosponge ''[[Samus anonymus]]'' (up to 50 m), [[hexactinellid]] ''Scleroplegma lanterna'' (~100–600 m), hexactinellid ''Aulocalyx irregularis'' (~550–915 m), lithistid demosponge ''Neoaulaxinia persicum'' (~500–1700 m)]] |
|||
[[File:Generalised food web for sponge reefs.jpg|thumb|upright=2.2|Generalised food web for sponge reefs<ref name= Archer2020>{{cite journal | last1=Archer | first1=Stephanie K. | last2=Kahn | first2=Amanda S. | last3=Thiess | first3=Mary | last4=Law | first4=Lauren | last5=Leys | first5=Sally P. | last6=Johannessen | first6=Sophia C. | last7=Layman | first7=Craig A. | last8=Burke | first8=Lily | last9=Dunham | first9=Anya | title=Foundation Species Abundance Influences Food Web Topology on Glass Sponge Reefs | journal=Frontiers in Marine Science | publisher=Frontiers Media SA | volume=7 | date=24 September 2020 | issn=2296-7745 | doi=10.3389/fmars.2020.549478| doi-access=free }} [[File:CC-BY icon.svg|50px]] Material was copied from this source, which is available under a [https://creativecommons.org/licenses/by/4.0/ Creative Commons Attribution 4.0 International License].</ref>]] |
|||
[[File:Generalised food web for sponge reefs.jpg|thumb|upright=2.2|Generalised food web for sponge reefs<ref name= Archer2020>{{cite journal |last1=Archer |first1=Stephanie K. |last2=Kahn |first2=Amanda S. |last3=Thiess |first3=Mary |last4=Law |first4=Lauren |last5=Leys |first5=Sally P. |last6=Johannessen |first6=Sophia C. |last7=Layman |first7=Craig A. |last8=Burke |first8=Lily |last9=Dunham |first9=Anya |title=Foundation Species Abundance Influences Food Web Topology on Glass Sponge Reefs |journal=Frontiers in Marine Science |publisher=Frontiers Media SA |volume=7 |date=24 September 2020 |issn=2296-7745 |doi=10.3389/fmars.2020.549478|doi-access=free }} [[File:CC-BY icon.svg|50px]] Material was copied from this source, which is available under a [https://creativecommons.org/licenses/by/4.0/ Creative Commons Attribution 4.0 International License] {{Webarchive|url=https://web.archive.org/web/20171016050101/https://creativecommons.org/licenses/by/4.0/ |date=2017-10-16 }}.</ref>]] |
|||
{{clear}} |
{{clear}} |
||
=== Sponge loop === |
=== Sponge loop === |
||
Most sponges are [[detritivore]]s which filter [[Particulate organic matter|organic debris particles]] and [[Marine microorganisms|microscopic life forms]] from ocean water. In particular, sponges occupy an important role as detritivores in [[coral reef food web]]s by recycling detritus to higher [[trophic level]]s.<ref name=Rix2018 /> |
|||
Most sponges are [[detritivore]]s which filter [[Particulate organic matter|organic debris particles]] and [[Marine microorganisms|microscopic life forms]] from ocean water. In particular, sponges occupy an important role as detritivores in [[coral reef food web]]s by recycling detritus to higher [[trophic level]]s.<ref name="Rix_2018"/> |
|||
The hypothesis has been made that coral reef sponges facilitate the transfer of coral-derived organic matter to their associated detritivores via the production of sponge detritus, as shown in the diagram. Several sponge species are able to convert coral-derived DOM into sponge detritus,<ref>Rix L, de Goeij JM, Mueller CE, Struck U and others (2016) "Coral mucus fuels the sponge loop in warm- and coldwater coral reef ecosystems". ''Sci Rep'', '''6''': 18715.</ref><ref>Rix L, de Goeij JM, van Oevelen D, Struck U, Al-Horani FA, Wild C, Naumann MS (2017) "Differential recycling of coral and algal dissolved organic matter via the sponge loop". ''Funct Ecol'' '''31''': 778−789.</ref> and transfer organic matter produced by corals further up the reef food web. Corals release organic matter as both dissolved and particulate mucus,<ref>Crossland CJ (1987) In situ release of mucus and DOC-lipid from the corals Acropora variabilis and Stylophora pistillata in different light regimes. Coral Reefs 6: 35−42</ref><ref name=Wild2004>Wild C, Huettel M, Klueter A, Kremb S, Rasheed M, Jorgensen B (2004) Coral mucus functions as an energy carrier and particle trap in the reef ecosystem. Nature 428: 66−70</ref><ref>Tanaka Y, Miyajima T, Umezawa Y, Hayashibara T, Ogawa H, Koike I (2009) Net release of dissolved organic matter by the scleractinian coral Acropora pulchra. J Exp Mar Biol Ecol 377: 101−106</ref><ref>Naumann M, Haas A, Struck U, Mayr C, El-Zibdah M, Wild C (2010) Organic matter release by dominant hermatypic corals of the Northern Red Sea. Coral Reefs 29: 649−659</ref> as well as cellular material such as expelled ''[[Symbiodinium]]''.<ref name=Hoegh-Guldberg1987>Hoegh-Guldberg O, McCloskey LR, Muscatine L (1987) Expulsion of zooxanthellae by symbiotic cnidarians from the Red Sea. Coral Reefs 5: 201−204</ref><ref>Baghdasarian G, Muscatine L (2000) "Preferential expulsion of dividing algal cells as a mechanism for regulating algal-cnidarian symbiosis". ''Biol Bull'', '''199''': 278−286</ref><ref name=Rix2018 /> |
|||
The hypothesis has been made that coral reef sponges facilitate the transfer of coral-derived organic matter to their associated detritivores via the production of sponge detritus, as shown in the diagram. Several sponge species are able to convert coral-derived DOM into sponge detritus,<ref>Rix L, de Goeij JM, Mueller CE, Struck U and others (2016) "Coral mucus fuels the sponge loop in warm- and coldwater coral reef ecosystems". ''Sci Rep'', '''6''': 18715.</ref><ref name="Rix_2017">{{cite journal |last1=Rix |first1=Laura |last2=de Goeij |first2=Jasper M. |last3=van Oevelen |first3=Dick |last4=Struck |first4=Ulrich |last5=Al-Horani |first5=Fuad A. |last6=Wild |first6=Christian |last7=Naumann |first7=Malik S. |date=March 2017 |title=Differential recycling of coral and algal dissolved organic matter via the sponge loop |journal=Functional Ecology |volume=31 |issue=3 |pages=778−789 |doi=10.1111/1365-2435.12758 |bibcode=2017FuEco..31..778R }}</ref> and transfer organic matter produced by corals further up the reef food web. Corals release organic matter as both dissolved and particulate mucus,<ref>{{cite journal |last= Crossland |first=C.J. |date=July 1987 |title=In situ release of mucus and DOC-lipid from the corals Acropora variabilis and Stylophora pistillata in different light regimes. |journal=Coral Reefs |volume=6 |issue=1 |pages=35−42 |doi=10.1007/BF00302210 |bibcode=1987CorRe...6...35C }}</ref><ref name="Wild_2004">{{cite journal |last1=Wild |first1=Christian |last2=Huettel |first2=Markus |last3=Klueter |first3=Anke |last4=Kremb |first4=Stephan G. |last5=Rasheed |first5=Mohammed Y. M. |last6=Jørgensen |first6=Bo B. |title=Coral mucus functions as an energy carrier and particle trap in the reef ecosystem |journal=Nature |volume=428 |issue=6978 |date=2004 |issn=0028-0836 |doi=10.1038/nature02344 |pages=66–70|pmid=14999280 |bibcode=2004Natur.428...66W }}</ref><ref>{{cite journal |last1=Tanaka |first1=Yasuaki |last2=Miyajima |first2=Toshihiro |last3=Umezawa |first3=Yu |last4=Hayashibara |first4=Takeshi |last5=Ogawa |first5=Hiroshi |last6=Koike |first6=Isao |title=Net release of dissolved organic matter by the scleractinian coral Acropora pulchra |journal=Journal of Experimental Marine Biology and Ecology |volume=377 |issue=2 |date=2009 |doi=10.1016/j.jembe.2009.06.023 |pages=101–106|bibcode=2009JEMBE.377..101T }}</ref><ref>{{cite journal |last1=Naumann |first1=M. S. |last2=Haas |first2=A. |last3=Struck |first3=U. |last4=Mayr |first4=C. |last5=el-Zibdah |first5=M. |last6=Wild |first6=C. |date=September 2010 |title=Organic matter release by dominant hermatypic corals of the Northern Red Sea. |journal=Coral Reefs |volume=29 |issue=3 |pages=649−659 |doi=10.1007/s00338-010-0612-7 |bibcode=2010CorRe..29..649N }}</ref> as well as cellular material such as expelled ''[[Symbiodinium]]''.<ref name="Hoegh-Guldberg_1987">{{cite journal |last1=Hoegh-Guldberg |first1=O. |last2=McCloskey |first2=L. R. |last3=Muscatine |first3=L. |date=April 1987 |title=Expulsion of zooxanthellae by symbiotic cnidarians from the Red Sea. |journal=Coral Reefs |volume=5 |issue=4 |pages=201−204 |doi=10.1007/BF00300964 |bibcode=1987CorRe...5..201H }}</ref><ref name="pmid11147708">{{cite journal |last1=Baghdasarian |first1=G |last2=Muscatine |first2=L |title=Preferential expulsion of dividing algal cells as a mechanism for regulating algal-cnidarian symbiosis |journal=The Biological Bulletin |volume=199 |issue=3 |date=2000 |issn=0006-3185 |doi=10.2307/1543184 |pages=278–286|jstor=1543184 |pmid=11147708 |url=https://www.biodiversitylibrary.org/part/10578 }}</ref><ref name="Rix_2018"/> |
|||
Organic matter could be transferred from corals to sponges by all these pathways, but DOM likely makes up the largest fraction, as the majority (56 to 80%) of coral mucus dissolves in the water column,<ref name=Wild2004 /> and coral loss of fixed carbon due to expulsion of ''Symbiodinium'' is typically negligible (0.01%)<ref name=Hoegh-Guldberg1987 /> compared with mucus release (up to ~40%).<ref>Crossland CJ, Barnes DJ, Borowitzka MA (1980) "Diurnal lipid and mucus production in the staghorn coral ''Acropora acuminata''". ''Mar Biol'', '''60''': 81−90.</ref><ref>Tremblay P, Grover R, Maguer JF, Legendre L, Ferrier-Pagès C (2012) "Autotrophic carbon budget in coral tissue:a new 13C-based model of photosynthate translocation." ''J Exp Biol'', '''215''': 1384−1393. {{doi|10.1242/jeb.065201}}.</ref> Coral-derived organic matter could also be indirectly transferred to sponges via bacteria, which can also consume coral mucus.<ref>Ferrier-Pagès C, Leclercq N, Jaubert J, Pelegri SP (2000) "Enhancement of pico- and nanoplankton growth by coral exudates". ''Aquat Microb Ecol'', '''21''': 203−209. {{doi|10.3354/ame021203}}.</ref><ref>Wild C, Niggl W, Naumann MS, Haas AF (2010) "Organic matter release by Red Sea coral reef organisms—potential effects on microbial activity and in situ O<sub>2</sub> availability". ''Mar Ecol Prog Ser'', '''411''': 61−71. {{doi|10.3354/meps08653}}.</ref><ref>Tanaka Y, Ogawa H, Miyajima T (2011) "Production and bacterial decomposition of dissolved organic matter in a fringing coral reef". ''J Oceanogr'', '''67''': 427−437. {{doi|10.1007/s10872-011-0046-z}}.</ref><ref name=Rix2018 /> |
|||
Organic matter could be transferred from corals to sponges by all these pathways, but DOM likely makes up the largest fraction, as the majority (56 to 80%) of coral mucus dissolves in the water column,<ref name="Wild_2004"/> and coral loss of fixed carbon due to expulsion of ''Symbiodinium'' is typically negligible (0.01%)<ref name="Hoegh-Guldberg_1987"/> compared with mucus release (up to ~40%).<ref>{{cite journal |last1=Crossland |first1=C. J. |last2=Barnes |first2=D. J. |last3=Borowitzka |first3=M. A. |title=Diurnal lipid and mucus production in the staghorn coral Acropora acuminata |journal=Marine Biology |volume=60 |issue=2–3 |date=1980 |issn=0025-3162 |doi=10.1007/BF00389151 |pages=81–90|bibcode=1980MarBi..60...81C }}</ref><ref name="pmid22442377">{{cite journal |last1=Tremblay |first1=Pascale |last2=Grover |first2=Renaud |last3=Maguer |first3=Jean François |last4=Legendre |first4=Louis |last5=Ferrier-Pagès |first5=Christine |title=Autotrophic carbon budget in coral tissue: a new 13C-based model of photosynthate translocation |journal=Journal of Experimental Biology |volume=215 |issue=8 |date=15 April 2012 |issn=1477-9145 |doi=10.1242/jeb.065201 |pages=1384–1393|pmid=22442377 }}</ref> Coral-derived organic matter could also be indirectly transferred to sponges via bacteria, which can also consume coral mucus.<ref>{{cite journal |last1=Ferrier-Pagès |first1=C |last2=Leclercq |first2=N |last3=Jaubert |first3=J |last4=Pelegrí |first4=Sp |title=Enhancement of pico- and nanoplankton growth by coral exudates |journal=Aquatic Microbial Ecology |volume=21 |date=2000 |issn=0948-3055 |doi=10.3354/ame021203 |pages=203–209}}</ref><ref>{{cite journal |last=Wild |first=C. |display-authors=etal |date=July 2010 |title=Organic matter release by Red Sea coral reef organisms—potential effects on microbial activity and in situ O<sub>2</sub> availability. |journal=Marine Ecology Progress Series |volume=411 |pages=61−71 |doi=10.3354/meps08653 |bibcode=2010MEPS..411...61W }}</ref><ref>{{cite journal |last1=Tanaka |first1=Yasuaki |last2=Ogawa |first2=Hiroshi |last3=Miyajima |first3=Toshihiro |title=Production and bacterial decomposition of dissolved organic matter in a fringing coral reef |journal=Journal of Oceanography |volume=67 |issue=4 |date=2011 |issn=0916-8370 |doi=10.1007/s10872-011-0046-z |pages=427–437|bibcode=2011JOce...67..427T }}</ref><ref name="Rix_2018"/> |
|||
[[File:Sponge loop pathway.png|thumb|upright=2|left|Sponge loop hypothesis. Steps of the sponge loop pathway: (1) corals and algae release exudates as [[dissolved organic matter]] (DOM), (2) sponges take up DOM, (3) sponges release detrital [[particulate organic matter]] (POM), (4) sponge detritus (POM) is taken up by sponge-associated and free-living [[detritivore]]s.<ref name=Rix2018>Rix, L., de Goeij, J.M., van Oevelen, D., Struck, U., Al-Horani, F.A., Wild, C. and Naumann, M.S. (2018) "Reef sponges facilitate the transfer of coral-derived organic matter to their associated fauna via the sponge loop". ''Marine Ecology Progress Series'', '''589''': 85–96. {{doi|10.3354/meps12443}}. [[File:CC-BY icon.svg|50px]] Material was copied from this source, which is available under a [https://creativecommons.org/licenses/by/4.0/ Creative Commons Attribution 4.0 International License].</ref><ref>Rix L, de Goeij JM, van Oevelen D, Struck U, Al-Horani FA, Wild C and Naumann MS (2017) "Differential recycling of coral and algal dissolved organic matter via the sponge loop". ''Funct Ecol'', '''31''': 778−789.</ref><ref>de Goeij JM, van Oevelen D, Vermeij MJA, Osinga R, Middelburg JJ, de Goeij AFPM and Admiraal W (2013) "Surviving in a marine desert: the sponge loop retains resources within coral reefs". ''Science'', '''342''': 108−110.</ref>]] |
|||
[[File:Sponge loop pathway.png|thumb|upright=2|left|Sponge loop hypothesis. Steps of the sponge loop pathway: (1) corals and algae release exudates as [[dissolved organic matter]] (DOM), (2) sponges take up DOM, (3) sponges release detrital [[particulate organic matter]] (POM), (4) sponge detritus (POM) is taken up by sponge-associated and free-living [[detritivore]]s.<ref name="Rix_2018">{{cite journal |last1=Rix |first1=L. |last2=de Goeij |first2=J.M. |last3=van Oevelen |first3=D. |last4=Struck |first4=U. |last5=Al-Horani |first5=F.A. |last6=Wild |first6=C. |last7=Naumann |first7=M.S. |title=Reef sponges facilitate the transfer of coral-derived organic matter to their associated fauna via the sponge loop |journal=Marine Ecology Progress Series |volume=589 |date=23 February 2018 |issn=0171-8630 |doi=10.3354/meps12443 |pages=85–96|bibcode=2018MEPS..589...85R }} [[File:CC-BY icon.svg|50px]] Material was copied from this source, which is available under a [https://creativecommons.org/licenses/by/4.0/ Creative Commons Attribution 4.0 International License] {{Webarchive|url=https://web.archive.org/web/20171016050101/https://creativecommons.org/licenses/by/4.0/ |date=2017-10-16 }}</ref><ref name="Rix_2017"/><ref name="pmid24092742">{{cite journal |last1=de Goeij |first1=Jasper M. |last2=van Oevelen |first2=Dick |last3=Vermeij |first3=Mark J. A. |last4=Osinga |first4=Ronald |last5=Middelburg |first5=Jack J. |last6=de Goeij |first6=Anton F. P. M. |last7=Admiraal |first7=Wim |title=Surviving in a Marine Desert: The Sponge Loop Retains Resources Within Coral Reefs |journal=Science |volume=342 |issue=6154 |date=4 October 2013 |issn=0036-8075 |doi=10.1126/science.1241981 |pages=108–110|bibcode=2013Sci...342..108D }}</ref>]] |
|||
[[File:The sponge holobiont.webp|thumb|upright=2|right|The sponge holobiont. The sponge [[holobiont]] is an example of the concept of nested ecosystems. Key functions carried out by the [[microbiome]] (colored arrows) influence holobiont functioning and, through cascading effects, subsequently influence community structure and ecosystem functioning. Environmental factors act at multiple scales to alter microbiome, holobiont, community, and ecosystem scale processes. Thus, factors that alter microbiome functioning can lead to changes at the holobiont, community, or even ecosystem level and vice versa, illustrating the necessity of considering multiple scales when evaluating functioning in nested ecosystems.<ref name=Pita2018>Pita, L., Rix, L., Slaby, B.M., Franke, A. and Hentschel, U. (2018) "The sponge holobiont in a changing ocean: from microbes to ecosystems". ''Microbiome'', '''6'''(1): 46. {{doi|10.1186/s40168-018-0428-1}}. [[File:CC-BY icon.svg|50px]] Material was copied from this source, which is available under a [https://creativecommons.org/licenses/by/4.0/ Creative Commons Attribution 4.0 International License].</ref> (DOM: [[dissolved organic matter]], POM: [[particulate organic matter]], DIN: dissolved inorganic nitrogen)]] |
|||
[[File:The sponge holobiont.webp|thumb|upright=2|right|The sponge holobiont. The sponge [[holobiont]] is an example of the concept of nested ecosystems. Key functions carried out by the [[microbiome]] (colored arrows) influence holobiont functioning and, through cascading effects, subsequently influence community structure and ecosystem functioning. Environmental factors act at multiple scales to alter microbiome, holobiont, community, and ecosystem scale processes. Thus, factors that alter microbiome functioning can lead to changes at the holobiont, community, or even ecosystem level and vice versa, illustrating the necessity of considering multiple scales when evaluating functioning in nested ecosystems.<ref name="Pita_2018">{{cite journal |last1=Pita |first1=L. |last2=Rix |first2=L. |last3=Slaby |first3=B. M. |last4=Franke |first4=A. |last5=Hentschel |first5=U. |title=The sponge holobiont in a changing ocean: from microbes to ecosystems |journal=Microbiome |volume=6 |issue=1 |date=2018 |page=46 |issn=2049-2618 |pmid=29523192 |pmc=5845141 |doi=10.1186/s40168-018-0428-1 |doi-access=free}} [[File:CC-BY icon.svg|50px]] Material was copied from this source, which is available under a [https://creativecommons.org/licenses/by/4.0/ Creative Commons Attribution 4.0 International License] {{Webarchive|url=https://web.archive.org/web/20171016050101/https://creativecommons.org/licenses/by/4.0/ |date=2017-10-16 }}.</ref> (DOM: [[dissolved organic matter]], POM: [[particulate organic matter]], DIN: dissolved inorganic nitrogen)]] |
|||
=== Sponge holobiont === |
=== Sponge holobiont === |
||
Besides a one to one [[symbiotic relationship]], it is possible for a host to become symbiotic with a [[microbial consortium]]. Sponges are able to host a wide range of [[Marine microorganisms|microbial communities]] that can also be very specific. The microbial communities that form a symbiotic relationship with the sponge can amount to as much as 35% of the [[Biomass (ecology)|biomass]] of its host.<ref>{{cite journal | vauthors = Egan S, Thomas T | title = Editorial for: Microbial symbiosis of marine sessile hosts- diversity and function | language = en | journal = Frontiers in Microbiology | volume = 6 | pages = 585 | date = 2015 | pmid = 26136729 | doi = 10.3389/fmicb.2015.00585 | pmc = 4468920 | doi-access = free }}</ref> The term for this specific symbiotic relationship, where a microbial consortia pairs with a host is called a [[Holobiont|holobiotic relationship]]. The sponge as well as the microbial community associated with it will produce a large range of secondary [[metabolite]]s that help protect it against predators through mechanisms such as [[chemical defense]].<ref name=Webster2016>{{cite journal | vauthors = Webster NS, Thomas T | title = The Sponge Hologenome | journal = mBio | volume = 7 | issue = 2 | pages = e00135-16 | date = April 2016 | pmid = 27103626 | doi = 10.1128/mBio.00135-16 | pmc = 4850255 }}</ref> |
|||
Besides a one to one [[symbiotic relationship]], it is possible for a host to become symbiotic with a [[microbial consortium]], resulting in a diverse [[Sponge microbiomes|sponge microbiome]]. Sponges are able to host a wide range of [[Marine microorganisms|microbial communities]] that can also be very specific. The microbial communities that form a symbiotic relationship with the sponge can amount to as much as 35% of the [[Biomass (ecology)|biomass]] of its host.<ref>{{cite journal |last1=Egan |first1=Suhelen |last2=Thomas |first2=Torsten |title=Editorial for: Microbial symbiosis of marine sessile hosts- diversity and function |journal=Frontiers in Microbiology |volume=6 |date=16 June 2015 |page=585 |issn=1664-302X |pmid=26136729 |pmc=4468920 |doi=10.3389/fmicb.2015.00585 |doi-access=free}}</ref> The term for this specific symbiotic relationship, where a microbial consortia pairs with a host is called a [[Holobiont|holobiotic relationship]]. The sponge as well as the microbial community associated with it will produce a large range of secondary [[metabolite]]s that help protect it against predators through mechanisms such as [[chemical defense]].<ref name="Webster_2016">{{cite journal |last1=Webster |first1=N.S. |last2=Thomas |first2=T. |title=The Sponge Hologenome |journal=mBio |volume=7 |issue=2 |pages=e00135-16 |date=April 2016 |pmid=27103626 |doi=10.1128/mBio.00135-16 |pmc=4850255 }}</ref> |
|||
Some of these relationships include endosymbionts within bacteriocyte cells, and cyanobacteria or microalgae found below the pinacoderm cell layer where they are able to receive the highest amount of light, used for phototrophy. They can host over 50 different microbial phyla and candidate phyla, including Alphaprotoebacteria, [[Actinomycetota]], [[Chloroflexota]], [[Nitrospirota]], "[[Cyanobacteria]]", the taxa Gamma-, the candidate phylum [[Poribacteria]], and [[Thaumarchaea]].<ref name=Webster2016 /> |
|||
Some of these relationships include endosymbionts within bacteriocyte cells, and cyanobacteria or microalgae found below the pinacoderm cell layer where they are able to receive the highest amount of light, used for phototrophy. They can host over 50 different microbial phyla and candidate phyla, including Alphaprotoebacteria, [[Actinomycetota]], [[Chloroflexota]], [[Nitrospirota]], "[[Cyanobacteria]]", the taxa Gamma-, the candidate phylum [[Poribacteria]], and [[Thaumarchaea]].<ref name="Webster_2016"/> |
|||
{{clear}} |
|||
== Systematics |
== Systematics == |
||
=== Taxonomy === |
=== Taxonomy === |
||
<!-- [[File:Sponge in papua new guinea.jpg|thumb|A sponge in [[Papua New Guinea]]]] --> |
<!-- [[File:Sponge in papua new guinea.jpg|thumb|A sponge in [[Papua New Guinea]]]] --> |
||
<!-- [[File:Biological classification L Pengo.svg|thumb|right|100px|Levels in the [[Linnean taxonomy]].]] --> |
<!-- [[File:Biological classification L Pengo.svg|thumb|right|100px|Levels in the [[Linnean taxonomy]].]] --> |
||
[[Carl Linnaeus|Linnaeus]], who classified most kinds of sessile animals as belonging to the order [[Vermes in the 10th edition of Systema Naturae#Zoophyta|Zoophyta]] in the class [[Vermes]], mistakenly identified the genus ''[[Spongia]]'' as plants in the order [[Algae]].<ref name=worms>{{cite web|url=http://www.marinespecies.org/aphia.php?p=sourceget&id=44036|title=Spongia Linnaeus, 1759|publisher=[[World Register of Marine Species]]|access-date=2012-07-18}}</ref> For a long time thereafter sponges were assigned to a separate subkingdom, [[Parazoa]] ("beside the animals"), separate from the [[Eumetazoa]] which formed the rest of the [[Kingdom (biology)|kingdom]] [[Animalia]].<ref name="Rowland2001ArchaeocyathaPhylogeneticInterpretations" /> They have been regarded as a [[paraphyly|paraphyletic]] [[phylum]], from which the higher animals have evolved.<ref>{{cite journal| vauthors = Sperling EA, Pisani D, Peterson KJ |title=Poriferan paraphyly and its implications for Precambrian palaeobiology |journal= Geological Society, London, Special Publications|date=January 1, 2007 |volume=286 |issue=1 |pages=355–368 |doi=10.1144/SP286.25 |url=http://www.dartmouth.edu/~peterson/Sperling,%20Pisani%20and%20Peterson.pdf |access-date=2012-08-22 |url-status=dead |archive-url=https://web.archive.org/web/20090509061759/http://www.dartmouth.edu/~peterson/Sperling,%20Pisani%20and%20Peterson.pdf |archive-date=May 9, 2009 |bibcode=2007GSLSP.286..355S |s2cid=34175521 }}</ref> Other research indicates Porifera is monophyletic.<ref>{{cite journal | vauthors = Whelan NV, Kocot KM, Moroz LL, Halanych KM | title = Error, signal, and the placement of Ctenophora sister to all other animals | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 112 | issue = 18 | pages = 5773–8 | date = May 2015 | pmid = 25902535 | pmc = 4426464 | doi = 10.1073/pnas.1503453112 | bibcode = 2015PNAS..112.5773W | doi-access = free }}</ref> |
|||
[[Carl Linnaeus]], who classified most kinds of sessile animals as belonging to the order [[Vermes in the 10th edition of Systema Naturae#Zoophyta|Zoophyta]] in the class [[Vermes]], mistakenly identified the genus ''[[Spongia]]'' as plants in the order [[Algae]].<ref name=worms>{{cite web |url=http://www.marinespecies.org/aphia.php?p=sourceget&id=44036 |title=Spongia Linnaeus, 1759 |publisher=[[World Register of Marine Species]] |access-date=18 July 2012 |archive-url=https://web.archive.org/web/20160327183441/http://www.marinespecies.org/aphia.php?p=sourceget&id=44036 |archive-date=27 March 2016 |url-status=live }}</ref>{{Explain |date=May 2024 |reason=Spongia 1st genus dedicated to sea sponges during time Linnaeus studied them? }} For a long time thereafter, sponges were assigned to subkingdom [[Parazoa]] ("beside the animals") separated from the [[Eumetazoa]] which formed the rest of the [[Kingdom (biology)|kingdom]] [[Animalia]].<ref name="Rowland_2001"/> |
|||
The phylum Porifera is further divided into [[Class (biology)|classes]] mainly according to the composition of their [[skeleton]]s:<ref name="HooperVanSoestDebrenne2002PhylumPorifera" /><ref name="Bergquist2001InEncOfLifeSci" /> |
|||
* [[Hexactinellida]] (glass sponges) have silicate spicules, the largest of which have six rays and may be individual or fused.<ref name="HooperVanSoestDebrenne2002PhylumPorifera" /> The main components of their bodies are [[syncytia]] in which large numbers of cell share a single external [[Cell membrane|membrane]].<ref name="Bergquist2001InEncOfLifeSci" /> |
|||
* [[Calcarea]] have skeletons made of [[calcite]], a form of [[calcium carbonate]], which may form separate spicules or large masses. All the cells have a single nucleus and membrane.<ref name="Bergquist2001InEncOfLifeSci" /> |
|||
* Most [[Demospongiae]] have silicate spicules or [[spongin]] fibers or both within their soft tissues. However, a few also have massive external skeletons made of [[aragonite]], another form of calcium carbonate.<ref name="HooperVanSoestDebrenne2002PhylumPorifera" /><ref name="Bergquist2001InEncOfLifeSci" /> All the cells have a single nucleus and membrane.<ref name="Bergquist2001InEncOfLifeSci" /> |
|||
* [[Archeocyatha]] are known only as fossils from the [[Cambrian]] period.<ref name="Rowland2001ArchaeocyathaPhylogeneticInterpretations" /> |
|||
The phylum Porifera is further divided into [[Class (biology)|classes]] mainly according to the composition of their [[skeleton]]s:<ref name="Hooper_2002"/><ref name="Bergquist_2001"/> |
|||
In the 1970s, sponges with massive calcium carbonate skeletons were assigned to a separate class, [[Sclerospongiae]], otherwise known as "coralline sponges".<ref>{{cite journal| vauthors = Hartman WD, Goreau TF |year=1970|title=Jamaican coralline sponges: Their morphology, ecology and fossil relatives|journal=Symposium of the Zoological Society of London|volume=25| pages=205–243}} [http://mgg.rsmas.miami.edu/groups/sil/work1.htm (cited by MGG.rsmas.miami.edu).] {{Webarchive|url=https://web.archive.org/web/20180818135926/http://mgg.rsmas.miami.edu/groups/sil/work1.htm |date=2018-08-18 }}</ref> |
|||
* [[Hexactinellida]] (glass sponges) have silicate spicules, the largest of which have six rays and may be individual or fused.<ref name="Hooper_2002"/> The main components of their bodies are [[syncytia]] in which large numbers of cell share a single external [[Cell membrane|membrane]].<ref name="Bergquist_2001"/> |
|||
However, in the 1980s it was found that these were all members of either the Calcarea or the Demospongiae.<ref>{{cite book| vauthors = Vacelet J |year=1985|contribution=Coralline sponges and the evolution of the Porifera|pages=1–13| veditors = Conway Morris S, George JD, Gibson R, Platt HM |title=The Origins and Relationships of Lower Invertebrates|publisher=[[Oxford University Press]]|isbn=978-0-19-857181-0}}</ref> |
|||
* [[Calcarea]] have skeletons made of [[calcite]], a form of [[calcium carbonate]], which may form separate spicules or large masses. All the cells have a single nucleus and membrane.<ref name="Bergquist_2001"/> |
|||
* Most [[Demospongiae]] have silicate spicules or [[spongin]] fibers or both within their soft tissues. However, a few also have massive external skeletons made of [[aragonite]], another form of calcium carbonate.<ref name="Hooper_2002"/><ref name="Bergquist_2001"/> All the cells have a single nucleus and membrane.<ref name="Bergquist_2001"/> |
|||
* [[Archeocyatha]] are known only as fossils from the [[Cambrian]] period.<ref name="Rowland_2001"/> |
|||
In the 1970s, sponges with massive calcium carbonate skeletons were assigned to a separate class, [[Sclerospongiae]], otherwise known as "coralline sponges".<ref>{{cite journal |last1=Hartman |first1=W.D. |last2=Goreau |first2=T.F. |year=1970|title=Jamaican coralline sponges: Their morphology, ecology and fossil relatives|journal=Symposium of the Zoological Society of London|volume=25|pages=205–243}} [http://mgg.rsmas.miami.edu/groups/sil/work1.htm (cited by MGG.rsmas.miami.edu).] {{Webarchive|url=https://web.archive.org/web/20180818135926/http://mgg.rsmas.miami.edu/groups/sil/work1.htm |date=2018-08-18 }}</ref> |
|||
So far scientific publications have identified about 9,000 poriferan species,<ref name="Bergquist2001InEncOfLifeSci" /> of which: about 400 are glass sponges; about 500 are calcareous species; and the rest are demosponges.<ref name="RuppertBarnes2004Porifera" /> However, some types of habitat, vertical rock and cave walls and galleries in rock and coral boulders, have been investigated very little, even in shallow seas.<ref name="Bergquist2001InEncOfLifeSci" /><!-- |
|||
However, in the 1980s it was found that these were all members of either the Calcarea or the Demospongiae.<ref>{{cite book |last=Vacelet |first=J. |year=1985 |contribution=Coralline sponges and the evolution of the Porifera |pages=1–13 |editor1=Conway Morris, S. |editor2=George, J.D. |editor3=Gibson, R. |editor4=Platt, H.M. |title=The Origins and Relationships of Lower Invertebrates |publisher=[[Oxford University Press]]|isbn=978-0-19-857181-0}}</ref> |
|||
********** old, keep in case any can be cannibalized |
|||
=== Basal lineage? === |
|||
So far scientific publications have identified about 9,000 poriferan species,<ref name="Bergquist_2001"/> of which: about 400 are glass sponges; about 500 are calcareous species; and the rest are demosponges.<ref name="Ruppert_2004"/> However, some types of habitat, vertical rock and cave walls and galleries in rock and coral boulders, have been investigated very little, even in shallow seas.<ref name="Bergquist_2001"/> |
|||
Sponges are among the simplest [[animals]]. They lack [[Gastrulation|gastrulated embryos]], [[Gastrointestinal tract|extracellular digestive cavities]], [[nerve]]s, [[muscle]]s, [[tissue (biology)|tissues]], and obvious sensory structures, features possessed by all other animals. In addition, sponge [[choanocyte]]s (feeding cells) appear to be a homologous to [[choanoflagellate]]s, a group of unicellular and colonial protists that are believed to be the immediate precursors of animals. The traditional conclusion is that sponges are the basal lineage of the animals, and that features such as tissues developed after sponges and other animals diverged. Sponges were first assigned their own subkingdom, the [[Parazoa]], but more recent molecular studies suggested that the sponges were [[paraphyletic]] to other animals, with the eumetazoa as a sister group to the most derived:<ref name=Sperling2007>{{cite journal| vauthors = Sperling EA Peterson KJ |year=2007|title=Poriferan paraphyly and its implications for Precambrian paleobiology|journal=Geological Society, London, Special Publications|url=http://sp.lyellcollection.org/cgi/content/abstract/286/1/355|doi=10.1144/SP286.25 |volume=286 |issue=1 |pages=355–368 |bibcode=2007GSLSP.286..355S}}</ref> ************* --> |
|||
=== Classes === |
=== Classes === |
||
Sponges were traditionally distributed in three classes: calcareous sponges (Calcarea), glass sponges (Hexactinellida) and demosponges (Demospongiae). However, studies have shown that the [[Homoscleromorpha]], a group thought to belong to the [[Demospongiae]], is actually phylogenetically well separated.{{sfn|Bergquist|1978|pp=153–154}} Therefore, they have recently been recognized as the fourth class of sponges.<ref name="Gazave2010">{{cite journal | vauthors = Gazave E, Lapébie P, Renard E, Vacelet J, Rocher C, Ereskovsky AV, Lavrov DV, Borchiellini C | title = Molecular phylogeny restores the supra-generic subdivision of homoscleromorph sponges (Porifera, Homoscleromorpha) | journal = PLOS ONE | volume = 5 | issue = 12 | pages = e14290 | date = December 2010 | pmid = 21179486 | pmc = 3001884 | doi = 10.1371/journal.pone.0014290 | bibcode = 2010PLoSO...514290G | doi-access = free }}</ref><ref name="Gazave2012">{{cite journal | vauthors = Gazave E, Lapébie P, Ereskovsky AV, Vacelet J, Renard E, Cárdenas P, Borchiellini C | date = May 2012 | title = No longer Demospongiae: Homoscleromorpha formal nomination as a fourth class of Porifera | url = https://hal.archives-ouvertes.fr/hal-01456632/file/Gazave%20et_2012-%20class.pdf| journal = Hydrobiologia | volume = 687 | pages = 3–10 | doi = 10.1007/s10750-011-0842-x | s2cid = 14468684 }}</ref> |
|||
Sponges were traditionally distributed in three classes: calcareous sponges (Calcarea), glass sponges (Hexactinellida) and demosponges (Demospongiae). However, studies have now shown that the [[Homoscleromorpha]], a group thought to belong to the [[Demospongiae]], has a [[phylogenetics|genetic relationship]] well separated from other sponge classes.<ref name="Bergquist_1978"/>{{rp|153–154}} Therefore, they have recently been recognized as the fourth class of sponges.<ref name="Gazave2010">{{cite journal |last1=Gazave |first1=Eve |last2=Lapébie |first2=Pascal |last3=Renard |first3=Emmanuelle |last4=Vacelet |first4=Jean |last5=Rocher |first5=Caroline |last6=Ereskovsky |first6=Alexander V. |last7=Lavrov |first7=Dennis V. |last8=Borchiellini |first8=Carole |title=Molecular phylogeny restores the supra-generic subdivision of homoscleromorph sponges (Porifera, Homoscleromorpha) |journal=PLOS ONE |volume=5 |issue=12 |pages=e14290 |date=December 2010 |pmid=21179486 |pmc=3001884 |doi=10.1371/journal.pone.0014290 |bibcode=2010PLoSO...514290G |doi-access=free }}</ref><ref name="Gazave_2012">{{cite journal |last1=Gazave |first1=Eve |last2=Lapébie |first2=Pascal |last3=Ereskovsky |first3=Alexander V. |last4=Vacelet |first4=Jean |last5=Renard |first5=Emmanuelle |last6=Cárdenas |first6=Paco |last7=Borchiellini |first7=Carole |date=May 2012 |title=No longer Demospongiae: Homoscleromorpha formal nomination as a fourth class of Porifera |url=https://hal.archives-ouvertes.fr/hal-01456632/file/Gazave%20et_2012-%20class.pdf |journal=Hydrobiologia |volume=687 |issue=1 |pages=3–10 |doi=10.1007/s10750-011-0842-x |bibcode=2012HyBio.687....3G |s2cid=14468684 |access-date=2019-08-01 |archive-date=2019-08-01 |archive-url=https://web.archive.org/web/20190801192211/https://hal.archives-ouvertes.fr/hal-01456632/file/Gazave%2520et_2012-%2520class.pdf |url-status=live }}</ref> |
|||
<!-- [[File:Sponge in papua new guinea.jpg|thumb|A sponge in [[Papua New Guinea]]]] --> |
<!-- [[File:Sponge in papua new guinea.jpg|thumb|A sponge in [[Papua New Guinea]]]] --> |
||
Sponges are divided into [[Class (biology)|classes]] mainly according to the composition of their [[skeleton]]s:<ref name=" |
Sponges are divided into [[Class (biology)|classes]] mainly according to the composition of their [[skeleton]]s:<ref name="Bergquist_1998"/> These are arranged in evolutionary order as shown below in ascending order of their evolution from top to bottom: |
||
:{| |
:{|class="wikitable" |
||
! [[Class (biology)|Class]] !! Type of cells<ref name=" |
! [[Class (biology)|Class]] !! Type of cells<ref name="Bergquist_1998">{{cite book |last=Bergquist |first=P.R. |year= 1998|chapter=Porifera|pages=10–27 |editor=Anderson, D.T. |title=Invertebrate Zoology|publisher=Oxford University Press|isbn=978-0-19-551368-4}}</ref> !! [[Sponge spicule|Spicules]]<ref name="Bergquist_1998"/> !! [[Spongin]] fibers<ref name="Bergquist_1998"/> !! Massive exoskeleton<ref name="Bergquist_2001"/> !! Body form<ref name="Bergquist_1998"/> |
||
|- |
|- |
||
! [[Hexactinellida]] |
! [[Hexactinellida]] |
||
| |
|Mostly [[syncytia]] in all species||[[Silica]]<br />May be individual or fused ||Never ||Never ||Leuconoid |
||
|- |
|- |
||
! [[Demospongiae]] |
! [[Demospongiae]] |
||
| |
|Single nucleus, single external membrane ||Silica ||In many species ||In some species.<br />Made of [[aragonite]] if present.<ref name="Hooper_2002"/><ref name="Bergquist_2001"/>||Leuconoid |
||
|- |
|- |
||
! [[Calcarea]] |
! [[Calcarea]] |
||
| |
|Single nucleus, single external membrane||[[Calcite]]<br />May be individual or large masses ||Never ||Common.<br />Made of calcite if present.||Asconoid, syconoid, leuconoid or solenoid<ref name="Cavalcanti_2012">{{cite journal |last1=Cavalcanti |first1=F.F. |last2=Klautau |first2=M. |year=2011 |title=Solenoid: a new aquiferous system to Porifera |journal=Zoomorphology |volume=130 |issue=4|pages=255–260 |doi=10.1007/s00435-011-0139-7 |s2cid=21745242 }}</ref> |
||
|- |
|- |
||
! [[Homoscleromorpha]] |
! [[Homoscleromorpha]] |
||
| |
|Single nucleus, single external membrane||Silica ||In many species ||Never ||Sylleibid or leuconoid |
||
|} |
|} |
||
=== Phylogeny === |
|||
The phylogeny of sponges has been debated heavily since the advent of [[phylogenetics]]. Originally thought to be the most basal animal phylum, there is now considerable evidence that [[Ctenophora]] may hold that title instead.<ref>{{cite book |last1=Giribet |first1=Gonzalo |last2=Edgecombe |first2=Gregory |title=The Invertebrate Tree of Life |date=3 March 2020 |publisher=Princeton University Press}}</ref><ref>{{cite journal |author1=Schultz, Darrin |display-authors=et al. |title=Ancient gene linkages support ctenophores as sister to other animals |url=https://www.nature.com/articles/s41586-023-05936-6?fromPaywallRec=false#citeas |journal=Nature |date=17 May 2023 |volume=618 |issue=7963 |pages=110–117 |doi=10.1038/s41586-023-05936-6|pmid=37198475 |bibcode=2023Natur.618..110S }}</ref> Additionally, the [[monophyly]] of the phylum is now under question. Several studies have concluded that [[Eumetazoa|all other animals]] emerged from within the sponges, and usually recover that the [[calcareous sponge]]s and [[Homoscleromorpha]] are closer to other animals than to [[demosponge]]s.<ref name="Sperling09">{{cite journal |last1=Sperling |first1=Erik |last2=Peterson |first2=Kevin |last3=Pisani |first3=David |title=Phylogenetic-Signal Dissection of Nuclear Housekeeping Genes Supports the Paraphyly of Sponges and the Monophyly of Eumetazoa |journal=Oxford Academic |date=October 2009 |volume=26 |issue=10 |pages=2261–2274 |doi=10.1093/molbev/msp148 |pmid=19597161 |url=https://academic.oup.com/mbe/article/26/10/2261/1109936?searchresult=1}}</ref><ref>{{cite journal |last1=Borchiellini |title=Sponge paraphyly and the origin of Metazoa |journal=Oxford Academic |date=1 January 2001 |volume=14 |issue=1 |pages=171–179 |doi=10.1046/j.1420-9101.2001.00244.x |pmid=29280585 |url=https://academic.oup.com/jeb/article/14/1/171/7323199?searchresult=1}}</ref> The internal relationships of Porifera have proven to be less uncertain. A close relationship of Homoscleromorpha and Calcarea has been recovered in nearly all studies, whether or not they support sponge or eumetazoan monophyly.<ref name="Sperling09"></ref><ref name="Feuda_2017"></ref><ref name="Gazave_2012"></ref><ref name="Gazave2010"></ref> The position of [[glass sponge]]s is also fairly certain, with a majority of studies recovering them as the sister of the demosponges.<ref name="Gazave2010"></ref><ref name="Feuda_2017"></ref><ref name="Sperling09"></ref> Thus, the uncertainty at the base of the animal family tree is probably best represented by the below cladogram. |
|||
{{clade |
|||
|label1=Animalia |
|||
|1={{clade |
|||
|label1= |
|||
|1={{clade |
|||
|label1= |
|||
|1='''[[Hexactinellid]]a''' [[File:Glass-sponge, Euplectella aspergillum.jpg|70px]] |
|||
|2='''[[Demospongiae]]''' [[File:Callyspongiatransparent.png||50px]] |
|||
}} |
|||
|2={{clade |
|||
|label1= |
|||
|1='''[[Calcarea]]''' [[File:Clathrina clathrus.jpg|55px]] |
|||
|2='''[[Homoscleromorpha]]''' [[File:Oscarella lobularis - Schmidt, 1862.jpg|55px]] |
|||
}} |
|||
|3=[[Ctenophora]] [[File:Mnemiopsis_leidyi_247259012.png|60px]] |
|||
|4=[[ParaHoxozoa]][[File:European_wasp_white_bg.jpg|60px]] |
|||
}} |
|||
}} |
|||
== Evolutionary history == |
|||
=== Fossil record === |
=== Fossil record === |
||
{{Redirect-distinguish|Primitive Sponge|Sponge (material)#History}} |
{{Redirect-distinguish|Primitive Sponge|Sponge (material)#History}} |
||
[[File:Raphidonema faringdonense 070715a.jpg|thumb|left|''Raphidonema faringdonense'', a fossil sponge from the [[Cretaceous]] of England]] |
|||
[[File:Raphidonema faringdonense 070715a.jpg|thumb|left|''[[Raphidonema (sponge)|Raphidonema faringdonense]]'', a fossil sponge from the [[Cretaceous]] of England]] |
|||
{{Annotated image |float=right|caption=[[Archaeocyathid]] structure|image=Archaeocyatha.png|width=150|image-width=150|height=235|image-top=-5|annotations= |
{{Annotated image |float=right|caption=[[Archaeocyathid]] structure|image=Archaeocyatha.png|width=150|image-width=150|height=235|image-top=-5|annotations= |
||
{{Annotation|4|11|'''1'''}} |
{{Annotation|4|11|'''1'''}} |
||
| Line 380: | Line 398: | ||
{{Annotation|2|175|'''1''': Gap '''2''': Central cavity '''3''' Internal wall '''4''': Pore (''all'' walls have pores) '''5''' Septum '''6''' Outer wall '''7''' Holdfast}} |
{{Annotation|2|175|'''1''': Gap '''2''': Central cavity '''3''' Internal wall '''4''': Pore (''all'' walls have pores) '''5''' Septum '''6''' Outer wall '''7''' Holdfast}} |
||
}} |
}} |
||
[[File:Nevadacoelia wistae.jpg|left|thumb|''[[Nevadacoelia|Nevadacoelia wistae]]'', a fossil [[Anthaspidellidae|anthaspidellid]] [[demosponge]] from the early [[Ordovician]] of [[Nevada]]. ]] |
|||
Although [[molecular clock]]s and [[biomarker]]s suggest sponges existed well before the [[Cambrian explosion]] of life, [[silica]] spicules like those of demosponges are absent from the fossil record until the Cambrian.<ref>{{cite journal | |
Although [[molecular clock]]s and [[biomarker]]s suggest sponges existed well before the [[Cambrian explosion]] of life, [[silica]] spicules like those of demosponges are absent from the fossil record until the Cambrian.<ref>{{cite journal |last1=Sperling |first1=E. A. |last2=Robinson |first2=J. M. |last3=Pisani |first3=D. |last4=Peterson |first4=K. J. |date=January 2010 |title=Where's the glass? Biomarkers, molecular clocks, and microRNAs suggest a 200 Myr missing Precambrian fossil record of siliceous sponge spicules |journal=Geobiology |volume=8 |issue=1 |pages=24–36 |pmid=19929965 |doi=10.1111/j.1472-4669.2009.00225.x |bibcode=2010Gbio....8...24S |s2cid=41195363 }}</ref> An unsubstantiated 2002 report exists of spicules in rocks dated around {{ma|750}}.<ref>{{cite book|last1= Reitner |first1=J |last2=Wörheide |first2=G. |contribution=Non-lithistid fossil Demospongiae – origins of their palaeobiodiversity and highlights in history of preservation |editor1=Hooper, J.N. |editor2=Van Soest, R.W. |year=2002 |title=Systema Porifera: A Guide to the Classification of Sponges |publisher=Kluwer Academic Plenum |location=New York, NY |url=http://webdoc.sub.gwdg.de/pub/geo/geobiologie/2005/reitner/2002-porifera.pdf |archive-url=https://web.archive.org/web/20081216220745/http://webdoc.sub.gwdg.de/pub/geo/geobiologie/2005/reitner/2002-porifera.pdf |archive-date=2008-12-16 |url-status=live |access-date=November 4, 2008}}</ref> Well-preserved [[fossil]] sponges from about {{ma|580}} in the [[Ediacaran]] period have been found in the [[Doushantuo Formation]].<ref>{{cite journal|last1=Li|first1=Chia-Wei|last2=Chen|first2=Jun-Yuan|last3=Hua|first3=Tzu-En|title=Precambrian sponges with cellular structures|journal=Science|date=February 1998|volume=279|issue=5352|pages=879–882|doi=10.1126/science.279.5352.879|pmid=9452391|bibcode=1998Sci...279..879L}}</ref> These fossils, which include: spicules; [[pinacocyte]]s; [[porocyte]]s; [[archeocyte]]s; [[sclerocyte]]s; and the internal cavity, have been classified as demosponges. Fossils of [[glass sponge]]s have been found from around {{ma|540}} in rocks in Australia, China, and Mongolia.<ref name="Müller_2007">{{cite journal |last=Müller |first=W. E. G. |author2=Jinhe Li |last3=Schröder |first3=H. C. |author4=Li Qiao |author5=Xiaohong Wang |year=2007 |title=The unique skeleton of siliceous sponges (Porifera; Hexactinellida and Demospongiae) that evolved first from the Urmetazoa during the Proterozoic: a review |journal=Biogeosciences |volume=4 |issue=2 |pages=219–232 |bibcode=2007BGeo....4..219M |doi=10.5194/bg-4-219-2007 |doi-access=free }}</ref> Early Cambrian sponges from Mexico belonging to the genus ''Kiwetinokia'' show evidence of fusion of several smaller spicules to form a single large spicule.<ref>{{cite journal |last= McMenamin |first=M.A. |year=2008 |title=Early Cambrian sponge spicules from the Cerro Clemente and Cerro Rajón, Sonora, México |journal=[[Geologica Acta]] |volume=6 |issue=4 |pages=363–367 }}</ref> [[Calcium carbonate]] spicules of [[Calcarea|calcareous sponges]] have been found in Early Cambrian rocks from about {{ma|530|523}} in Australia. Other probable demosponges have been found in the Early [[Cambrian]] [[Chengjiang fauna]], from {{ma|525|520}}.<ref name="Li_1998">{{cite journal |last1=Li |first1=Chia-Wei |last2=Chen |first2=Jun-Yuan |last3=Hua |first3=Tzu-En |date=February 1998 |title=Precambrian sponges with cellular structures |journal=Science |volume=279 |issue=5352 |pages=879–82 |pmid=9452391 |doi=10.1126/science.279.5352.879 |bibcode=1998Sci...279..879L |s2cid=38837724 }}</ref> Fossils found in the Canadian Northwest Territories dating to {{ma|890}} may be sponges; if this finding is confirmed, it suggests the first animals appeared before the Neoproterozoic oxygenation event.<ref name="Turner_2021">{{cite journal |last=Turner |first=E.C. |title=Possible poriferan body fossils in early Neoproterozoic microbial reefs |journal=Nature |volume=596 |issue=7870 |pages=87–91 |date=August 2021 |pmid=34321662 |pmc=8338550 |doi=10.1038/s41586-021-03773-z |doi-access=free |bibcode=2021Natur.596...87T }}</ref> |
||
[[File:Sauerstoffgehalt-1000mj2.png|thumb|left|Oxygen content of the atmosphere over the last billion years. If confirmed, the discovery of fossilized sponges dating to 890 million years ago would predate the Neoproterozoic Oxygenation Event.]] |
[[File:Sauerstoffgehalt-1000mj2.png|thumb|left|Oxygen content of the atmosphere over the last billion years. If confirmed, the discovery of fossilized sponges dating to 890 million years ago would predate the Neoproterozoic Oxygenation Event.]] |
||
Freshwater sponges appear to be much younger, as the earliest known fossils date from the Mid-[[Eocene]] period about {{ma|48|40}}.<ref name=" |
Freshwater sponges appear to be much younger, as the earliest known fossils date from the Mid-[[Eocene]] period about {{ma|48|40}}.<ref name="Müller_2007"/> Although about 90% of modern sponges are [[demosponges]], fossilized remains of this type are less common than those of other types because their skeletons are composed of relatively soft spongin that does not fossilize well.<ref name="Kazmierczak_2004">{{cite web |title=Demospongia |publisher=[[University of California, Berkeley|U.C. Berkeley]] |department=[[University of California Museum of Paleontology]] |place=Berkeley, CA |url=http://www.ucmp.berkeley.edu/porifera/demospongia.html |access-date=2008-11-27 |url-status=live |archive-url=https://web.archive.org/web/20131018230738/http://www.ucmp.berkeley.edu/porifera/demospongia.html |archive-date=October 18, 2013}}</ref> |
||
The earliest sponge symbionts are known from the [[Llandovery epoch|early Silurian]].<ref name=" |
The earliest sponge symbionts are known from the [[Llandovery epoch|early Silurian]].<ref name="Vinn_2015">{{cite journal |last1=Vinn |first1=Olev |last2=Wilson |first2=Mark A. |last3=Toom |first3=Ursula |last4=Mõtus |first4=Mari-Ann |year=2015 |title=Earliest known rugosan-stromatoporoid symbiosis from the Llandovery of Estonia (Baltica) |journal=Palaeogeography, Palaeoclimatology, Palaeoecology |volume=31 |pages=1–5 |bibcode=2015PPP...431....1V |doi=10.1016/j.palaeo.2015.04.023 |url=https://www.researchgate.net/publication/275657612 |access-date=2015-06-18 |archive-date=2024-04-10 |archive-url=https://web.archive.org/web/20240410181408/https://www.researchgate.net/publication/275657612_Earliest_known_rugosan-stromatoporoid_symbiosis_from_the_Llandovery_of_Estonia_Baltica |url-status=live }}</ref> |
||
A chemical tracer is 24-isopropyl[[cholestane]], which is a stable derivative of 24-isopropyl[[cholesterol]], which is said to be produced by [[demosponge]]s but not by [[eumetazoa]]ns ("true animals", i.e. [[cnidaria]]ns and [[bilateria]]ns). Since [[choanoflagellate]]s are thought to be animals' closest single-celled relatives, a team of scientists examined the [[biochemistry]] and [[gene]]s of one [[choanoflagellate]] species. They concluded that this species could not produce 24-isopropylcholesterol but that investigation of a wider range of choanoflagellates would be necessary in order to prove that the fossil 24-isopropylcholestane could only have been produced by demosponges.<ref>{{cite journal | |
A chemical tracer is 24-isopropyl[[cholestane]], which is a stable derivative of 24-isopropyl[[cholesterol]], which is said to be produced by [[demosponge]]s but not by [[eumetazoa]]ns ("true animals", i.e. [[cnidaria]]ns and [[bilateria]]ns). Since [[choanoflagellate]]s are thought to be animals' closest single-celled relatives, a team of scientists examined the [[biochemistry]] and [[gene]]s of one [[choanoflagellate]] species. They concluded that this species could not produce 24-isopropylcholesterol but that investigation of a wider range of choanoflagellates would be necessary in order to prove that the fossil 24-isopropylcholestane could only have been produced by demosponges.<ref>{{cite journal |last1=Kodner |first1=Robin B. |last2=Summons |first2=Roger E. |last3=Pearson |first3=Ann |last4=King |first4=Nicole |last5=Knoll |first5=Andrew H. |date=July 2008 |title=Sterols in a unicellular relative of the metazoans |journal=Proceedings of the National Academy of Sciences of the United States of America |volume=105 |issue=29 |pages=9897–9902 |pmid=18632573 |pmc=2481317 |doi=10.1073/pnas.0803975105 |bibcode=2008PNAS..105.9897K |doi-access=free }}</ref> |
||
Although a previous publication reported traces of the chemical 24-isopropyl[[cholestane]] in ancient rocks dating to {{ma|1800}},<ref>{{cite journal | |
Although a previous publication reported traces of the chemical 24-isopropyl[[cholestane]] in ancient rocks dating to {{ma|1800}},<ref>{{cite journal |last1=Nichols |first1=S. |last2=Wörheide |first2=G. |date=April 2005 |title=Sponges: New views of old animals |journal=Integrative and Comparative Biology |volume=45 |issue=2 |pages=333–334 |pmid=21676777 |doi=10.1093/icb/45.2.333 |citeseerx=10.1.1.598.4999 }}</ref> recent research using a much more accurately dated rock series has revealed that these biomarkers only appear before the end of the [[Marinoan glaciation]] approximately {{ma|635}},<ref>{{cite journal |last1=Love |first1=Gordon D. |last2=Grosjean |first2=Emmanuelle |last3=Stalvies |first3=Charlotte |last4=Fike |first4=David A. |last5=Grotzinger |first5=John P. |last6=Bradley |first6=Alexander S. |last7=Kelly |first7=Amy E. |last8=Bhatia |first8=Maya |last9=Meredith |first9=William |last10=Snape |first10=Colin E. |last11=Bowring |first11=Samuel A. |last12=Condon |first12=Daniel J. |last13=Summons |first13=Roger E. |display-authors=6 |date=February 2009 |title=Fossil steroids record the appearance of Demospongiae during the Cryogenian period |journal=Nature |volume=457 |issue=7230 |pages=718–721 |pmid=19194449 |doi=10.1038/nature07673 |bibcode=2009Natur.457..718L |s2cid=4314662 |url=https://authors.library.caltech.edu/14867/2/Love2009p34510.1038nature07673_supp.pdf |access-date=2019-08-01 |archive-url=https://web.archive.org/web/20180724144041/https://authors.library.caltech.edu/14867/2/Love2009p34510.1038nature07673_supp.pdf |archive-date=2018-07-24 |url-status=dead }}</ref> and that "Biomarker analysis has yet to reveal any convincing evidence for ancient sponges pre-dating the first globally extensive Neoproterozoic glacial episode (the Sturtian, ~{{ma|713}} in Oman)". While it has been argued that this 'sponge biomarker' could have originated from marine algae, recent research suggests that the algae's ability to produce this biomarker evolved only in the [[Carboniferous]]; as such, the biomarker remains strongly supportive of the presence of demosponges in the Cryogenian.<ref name="Antcliffe_2013">{{cite journal |author=Antcliffe, J.B. |year=2013 |editor=Stouge, S. |title=Questioning the evidence of organic compounds called sponge biomarkers |journal=Palaeontology |volume=56 |issue=5 |pages=917–925 |doi=10.1111/pala.12030 |doi-access=free |bibcode=2013Palgy..56..917A }}</ref><ref>{{cite journal |author=Gold, D.A. |title=The slow rise of complex life as revealed through biomarker genetics |journal=Emerging Topics in Life Sciences |volume=2 |issue=2 |pages=191–199 |date=September 2018 |pmid=32412622 |doi=10.1042/ETLS20170150 |s2cid=90887224 }}</ref><ref>{{cite journal |last1=Gold |first1=David A. |last2=Grabenstatter |first2=Jonathan |last3=de Mendoza |first3=Alex |last4=Riesgo |first4=Ana |last5=Ruiz-Trillo |first5=Iñaki |last6=Summons |first6=Roger E. |date=March 2016 |title=Sterol and genomic analyses validate the sponge biomarker hypothesis |journal=Proceedings of the National Academy of Sciences of the United States of America |volume=113 |issue=10 |pages=2684–2689 |pmid=26903629 |pmc=4790988 |doi=10.1073/pnas.1512614113 |bibcode=2016PNAS..113.2684G |doi-access=free }}</ref> |
||
[[Archaeocyathid]]s, which some classify as a type of coralline sponge, are very common fossils in rocks from the Early [[Cambrian]] about {{ma|530|520}}, but apparently died out by the end of the Cambrian {{ma|490}}.<ref name=" |
[[Archaeocyathid]]s, which some classify as a type of coralline sponge, are very common fossils in rocks from the Early [[Cambrian]] about {{ma|530|520}}, but apparently died out by the end of the Cambrian {{ma|490}}.<ref name="Li_1998"/> |
||
It has been suggested that they were produced by: sponges; [[cnidaria]]ns; [[algae]]; [[foraminifera]]ns; a completely separate [[phylum]] of animals, Archaeocyatha; or even a completely separate [[Kingdom (biology)|kingdom]] of life, labeled Archaeata or Inferibionta. Since the 1990s archaeocyathids have been regarded as a distinctive group of sponges.<ref name=" |
It has been suggested that they were produced by: sponges; [[cnidaria]]ns; [[algae]]; [[foraminifera]]ns; a completely separate [[phylum]] of animals, Archaeocyatha; or even a completely separate [[Kingdom (biology)|kingdom]] of life, labeled Archaeata or Inferibionta. Since the 1990s archaeocyathids have been regarded as a distinctive group of sponges.<ref name="Rowland_2001">{{cite journal |author1= Rowland, S.M. |author2=Stephens, T. |year=2001 |title=Archaeocyatha: A history of phylogenetic interpretation |journal=Journal of Paleontology |volume=75 |issue=6 |pages=1065–1078 |jstor=1307076 |doi=10.1666/0022-3360(2001)075<1065:AAHOPI>2.0.CO;2}}</ref> |
||
{{clear left}} |
{{clear left}} |
||
{{Annotated image|float=left|caption=[[Halkieriid]] sclerite structure<ref name=" |
{{Annotated image|float=left|caption=[[Halkieriid]] sclerite structure<ref name="Porter_2008"/> |image=Halkieriid sclerite structure 300.png |width=210 |height=96 |image-width=200 |image-left=0 |image-top=0 |
||
| |
|annotations = |
||
{{Annotation|141|44| |
{{Annotation|141|44|{{=}} skin}} |
||
{{Annotation|141|64| |
{{Annotation|141|64|{{=}} [[aragonite]]}} |
||
{{Annotation|141|84| |
{{Annotation|141|84|{{=}} flesh}} |
||
}} |
}} |
||
It is difficult to fit [[chancelloriid]]s into classifications of sponges or more complex animals. An analysis in 1996 concluded that they were closely related to sponges on the grounds that the detailed structure of chancellorid sclerites ("armor plates") is similar to that of fibers of spongin, a [[collagen]] [[protein]], in modern keratose (horny) [[demosponge]]s such as ''[[Darwinella (sponge)|Darwinella]]''.<ref>{{cite journal | |
It is difficult to fit [[chancelloriid]]s into classifications of sponges or more complex animals. An analysis in 1996 concluded that they were closely related to sponges on the grounds that the detailed structure of chancellorid sclerites ("armor plates") is similar to that of fibers of spongin, a [[collagen]] [[protein]], in modern keratose (horny) [[demosponge]]s such as ''[[Darwinella (sponge)|Darwinella]]''.<ref>{{cite journal |author1=Butterfield, N.J. |author2=Nicholas, C.J. |year=1996 |title=Burgess Shale-type preservation of both non-mineralizing and "shelly" Cambrian organisms from the Mackenzie Mountains, northwestern Canada |journal=Journal of Paleontology |volume=70 |issue=6 |pages=893–899 |jstor=1306492 |doi=10.1017/S0022336000038579 |bibcode=1996JPal...70..893B |s2cid=133427906 }}</ref> However, another analysis in 2002 concluded that chancelloriids are not sponges and may be intermediate between sponges and more complex animals, among other reasons because their skins were thicker and more tightly connected than those of sponges.<ref name="Janussen_2002">{{cite journal |last1=Janussen |first1=Dorte |last2=Steiner |first2=Michael |last3=Maoyan |first3=Zhu |year=2002 |title=New well-preserved scleritomes of Chancelloridae from the early Cambrian Yuanshan Formation (Chengjiang, China) and the middle Cambrian Wheeler Shale (Utah, USA) and paleobiological implications |journal=Journal of Paleontology |volume=76 |issue=4 |pages=596–606 |doi=10.1666/0022-3360(2002)076<0596:NWPSOC>2.0.CO;2|bibcode=2002JPal...76..596J |s2cid=129127213 }} free text at {{cite news |last=Janussen |first=D. |year=2002 |title=(full text without images) |journal=[[Journal of Paleontology]] |url=http://findarticles.com/p/articles/mi_qa3790/is_200207/ai_n9134583/pg_1?tag=artBody;col1 |access-date=2008-08-04 |archive-date=December 10, 2008 |url-status=dead |archive-url=https://web.archive.org/web/20081210092130/http://findarticles.com/p/articles/mi_qa3790/is_200207/ai_n9134583/pg_1?tag=artBody%3Bcol1}}</ref> In 2008, a detailed analysis of chancelloriids' sclerites concluded that they were very similar to those of [[halkieriid]]s, mobile [[bilaterian]] animals that looked like [[slug]]s in [[chain mail]] and whose fossils are found in rocks from the very Early Cambrian to the Mid Cambrian. If this is correct, it would create a dilemma, as it is extremely unlikely that totally unrelated organisms could have developed such similar sclerites independently, but the huge difference in the structures of their bodies makes it hard to see how they could be closely related.<ref name="Porter_2008">{{cite journal |last=Porter |first=S.M. |year=2008 |title=Skeletal microstructure indicates Chancelloriids and Halkieriids are closely related |journal=[[Palaeontology (journal)|Palaeontology]] |volume=51 |issue=4 |pages=865–879 |doi=10.1111/j.1475-4983.2008.00792.x |bibcode=2008Palgy..51..865P |doi-access=free}}</ref> |
||
=== Relationships to other animal groups === |
=== Relationships to other animal groups === |
||
| Line 406: | Line 424: | ||
{{cladogram |
{{cladogram |
||
|title=Simplified family tree showing [[Calcarea|calcareous sponges]] as closest to more complex animals<ref name="Borchiellini_2001"/> |
|||
|clades={{clade |
|||
|label1= [[Opisthokonta]] |
|||
|1={{clade |
|||
|1=[[Fungi]] |
|||
|2={{clade |
|||
|1=[[Choanoflagellate]]s |
|||
|label2= [[Metazoa]] |
|||
|2={{clade |
|||
|1=[[Glass sponge]]s |
|||
|2={{clade |
|||
|1=[[Demosponge]]s |
|||
|2={{clade |
|||
|1=[[Calcarea|Calcareous sponges]] |
|||
|label2= [[Eumetazoa]] |
|||
|2={{clade |
|2={{clade |
||
|1=[[Ctenophora|Comb jellies]] |
|||
|2={{clade |
|||
|1=[[Placozoa]] |
|||
|2={{clade |
|2={{clade |
||
|1=[[Cnidaria]]<br/>(jellyfish, etc.) |
|||
|2=other [[metazoa]]ns |
|||
}} |
|||
|2={{clade |
|||
|1=[[Calcarea|Calcareous sponges]] |
|||
|label2= [[Eumetazoa]] |
|||
|2={{clade |
|||
|1=[[Ctenophora|Comb jellies]] |
|||
|2={{clade |
|||
|1=[[Placozoa]] |
|||
|2={{clade |
|||
|1=[[Cnidaria]]<br/>(jellyfish, etc.) |
|||
|2=other [[metazoa]]ns |
|||
}} |
|||
}} |
|||
}} |
|||
}} |
|||
}} |
|||
}} |
|||
}} |
}} |
||
}} |
|||
}} |
}} |
||
}} |
|||
}} |
}} |
||
}} |
|||
}} |
|||
}} |
|||
}} |
}} |
||
{{cladogram |
{{cladogram |
||
|title=Simplified family tree showing [[Homoscleromorpha]] as closest to more complex animals<ref name="Sperling_2007"/> |
|||
|clades={{clade |
|||
|label1= [[Eukaryotes]] |
|||
|1={{clade |
|||
|1=[[Plant]]s |
|||
|2=[[Fungi]] |
|||
|label3= [[Metazoa]] |
|||
|3={{clade |
|||
|1=Most [[demosponge]]s |
|||
|2={{clade |
|||
|1=[[Calcarea|Calcareous sponges]] |
|||
|2={{clade |
|||
|1=[[Homoscleromorpha]] |
|||
|label2= [[Eumetazoa]] |
|||
|2={{clade |
|||
|1=[[Cnidaria]]<br/>(jellyfish, etc.) |
|||
|2=other [[metazoa]]ns |
|||
}} |
|||
}} |
|||
}} |
|||
}} |
|||
}} |
}} |
||
}} |
|||
}} |
}} |
||
}} |
|||
}} |
|||
}} |
|||
}} |
}} |
||
In the 1990s, sponges were widely regarded as a [[monophyletic]] group, all of them having descended from a [[last universal common ancestor|common ancestor]] that was itself a sponge, and as the "sister-group" to all other [[animals|metazoans]] (multi-celled animals), which themselves form a monophyletic group. On the other hand, some 1990s analyses also revived the idea that animals' nearest evolutionary relatives are [[choanoflagellate]]s, single-celled organisms very similar to sponges' [[choanocytes]] – which would imply that most Metazoa evolved from very sponge-like ancestors and therefore that sponges may not be monophyletic, as the same sponge-like ancestors may have given rise both to modern sponges and to non-sponge members of Metazoa.<ref name=" |
In the 1990s, sponges were widely regarded as a [[monophyletic]] group, all of them having descended from a [[last universal common ancestor|common ancestor]] that was itself a sponge, and as the "sister-group" to all other [[animals|metazoans]] (multi-celled animals), which themselves form a monophyletic group. On the other hand, some 1990s analyses also revived the idea that animals' nearest evolutionary relatives are [[choanoflagellate]]s, single-celled organisms very similar to sponges' [[choanocytes]] – which would imply that most Metazoa evolved from very sponge-like ancestors and therefore that sponges may not be monophyletic, as the same sponge-like ancestors may have given rise both to modern sponges and to non-sponge members of Metazoa.<ref name="Borchiellini_2001">{{cite journal |last1=Borchiellini |first1=C. |last2=Manuel |first2=M. |last3=Alivon |first3=E. |last4=Boury-Esnault |first4=N. |last5=Vacelet |first5=J. |last6=Le Parco |first6=Y. |title=Sponge paraphyly and the origin of Metazoa |journal=Journal of Evolutionary Biology |volume=14 |issue=1 |pages=171–179 |date=January 2001 |pmid=29280585 |doi=10.1046/j.1420-9101.2001.00244.x |doi-access=free }}</ref> |
||
Analyses since 2001 have concluded that [[Eumetazoa]] (more complex than sponges) are more closely related to particular groups of sponges than to other sponge groups. Such conclusions imply that sponges are not monophyletic, because the [[most recent common ancestor|last common ancestor]] of all sponges would also be a direct ancestor of the Eumetazoa, which are not sponges. A study in 2001 based on comparisons of [[ribosome]] [[DNA]] concluded that the most fundamental division within sponges was between [[glass sponge]]s and the rest, and that Eumetazoa are more closely related to [[Calcarea|calcareous sponges]] (those with calcium carbonate spicules) than to other types of sponge.<ref name=" |
Analyses since 2001 have concluded that [[Eumetazoa]] (more complex than sponges) are more closely related to particular groups of sponges than to other sponge groups. Such conclusions imply that sponges are not monophyletic, because the [[most recent common ancestor|last common ancestor]] of all sponges would also be a direct ancestor of the Eumetazoa, which are not sponges. A study in 2001 based on comparisons of [[ribosome]] [[DNA]] concluded that the most fundamental division within sponges was between [[glass sponge]]s and the rest, and that Eumetazoa are more closely related to [[Calcarea|calcareous sponges]] (those with calcium carbonate spicules) than to other types of sponge.<ref name="Borchiellini_2001"/> In 2007, one analysis based on comparisons of [[RNA]] and another based mainly on comparison of spicules concluded that demosponges and glass sponges are more closely related to each other than either is to the calcareous sponges, which in turn are more closely related to Eumetazoa.<ref name="Müller_2007"/><ref>{{cite journal |last1=Medina |first1=Mónica |last2=Collins |first2=Allen G. |last3=Silberman |first3=Jeffrey D. |last4=Sogin |first4=Mitchell L. |title=Evaluating hypotheses of basal animal phylogeny using complete sequences of large and small subunit rRNA |journal=Proceedings of the National Academy of Sciences of the United States of America |volume=98 |issue=17 |pages=9707–12 |date=August 2001 |pmid=11504944 |pmc=55517 |bibcode=2001PNAS...98.9707M |doi=10.1073/pnas.171316998 |doi-access=free }}</ref> |
||
Other anatomical and biochemical evidence links the Eumetazoa with [[Homoscleromorpha]], a sub-group of demosponges. A comparison in 2007 of [[cell nucleus|nuclear]] [[DNA]], excluding glass sponges and [[Ctenophora|comb jellies]], concluded that: |
Other anatomical and biochemical evidence links the Eumetazoa with [[Homoscleromorpha]], a sub-group of demosponges. A comparison in 2007 of [[cell nucleus|nuclear]] [[DNA]], excluding glass sponges and [[Ctenophora|comb jellies]], concluded that: |
||
| Line 471: | Line 489: | ||
* calcareous sponges are the next closest; |
* calcareous sponges are the next closest; |
||
* the other demosponges are evolutionary "aunts" of these groups; and |
* the other demosponges are evolutionary "aunts" of these groups; and |
||
* the [[chancelloriidae|chancelloriids]], bag-like animals whose fossils are found in [[Cambrian]] rocks, may be sponges.<ref name=" |
* the [[chancelloriidae|chancelloriids]], bag-like animals whose fossils are found in [[Cambrian]] rocks, may be sponges.<ref name="Sperling_2007">{{cite journal |last1=Sperling |first1=E. A. |last2=Pisani |first2=D. |last3=Peterson |first3=K. J. |year=2007 |title=Poriferan paraphyly and its implications for Precambrian paleobiology |journal=Journal of the Geological Society of London |volume=286 |issue=1 |pages=355–368 |doi=10.1144/SP286.25 |bibcode=2007GSLSP.286..355S |s2cid=34175521 |url=http://www.dartmouth.edu/~peterson/Sperling,%20Pisani%20and%20Peterson.pdf |url-status=dead |access-date=2008-11-04 |archive-url=https://web.archive.org/web/20090509061759/http://www.dartmouth.edu/~peterson/Sperling,%20Pisani%20and%20Peterson.pdf |archive-date=May 9, 2009}}</ref> |
||
The [[sperm]] of Homoscleromorpha share features with the sperm of Eumetazoa, that sperm of other sponges lack. In both Homoscleromorpha and Eumetazoa layers of cells are bound together by attachment to a carpet-like basal membrane composed mainly of "typ IV" [[collagen]], a form of collagen not found in other sponges – although the [[spongin]] fibers that reinforce the mesohyl of all demosponges is similar to "type IV" collagen.<ref name=" |
The [[sperm]] of Homoscleromorpha share features with the sperm of Eumetazoa, that sperm of other sponges lack. In both Homoscleromorpha and Eumetazoa layers of cells are bound together by attachment to a carpet-like basal membrane composed mainly of "typ IV" [[collagen]], a form of collagen not found in other sponges – although the [[spongin]] fibers that reinforce the mesohyl of all demosponges is similar to "type IV" collagen.<ref name="Exposito_2002">{{cite journal |last1=Exposito |first1=Jean-Yves |last2=Cluzel |first2=Caroline |last3=Garrone |first3=Robert |last4=Lethias |first4=Claire |title=Evolution of collagens |journal=The Anatomical Record |volume=268 |issue=3 |pages=302–16 |date=November 2002 |pmid=12382326 |doi=10.1002/ar.10162 |doi-access=free }}</ref> |
||
[[File:Bathocyroe fosteri.jpg|thumb|left|A [[ctenophora|comb jelly]] ]] |
[[File:Bathocyroe fosteri.jpg|thumb|left|A [[ctenophora|comb jelly]] ]] |
||
The analyses described above concluded that sponges are closest to the ancestors of all Metazoa, of all multi-celled animals including both sponges and more complex groups. However, another comparison in 2008 of 150 genes in each of 21 genera, ranging from fungi to humans but including only two species of sponge, suggested that [[ctenophora|comb jellies]] ([[ctenophora]]) are the most basal lineage of the Metazoa included in the sample.<ref name="Dunn_2008">{{cite journal | |
The analyses described above concluded that sponges are closest to the ancestors of all Metazoa, of all multi-celled animals including both sponges and more complex groups. However, another comparison in 2008 of 150 genes in each of 21 genera, ranging from fungi to humans but including only two species of sponge, suggested that [[ctenophora|comb jellies]] ([[ctenophora]]) are the most basal lineage of the Metazoa included in the sample.<ref name="Dunn_2008">{{cite journal |last1=Dunn |first1=Casey W. |last2=Hejnol |first2=Andreas |last3=Matus |first3=David Q. |last4=Pang |first4=Kevin |last5=Browne |first5=William E. |last6=Smith |first6=Stephen A. |last7=Seaver |first7=Elaine |last8=Rouse |first8=Greg W. |last9=Obst |first9=Matthias |last10=Edgecombe |first10=Gregory D. |last11=Sørensen |first11=Martin V. |last12=Haddock |first12=Steven H. D. |last13=Schmidt-Rhaesa |first13=Andreas |last14=Okusu |first14=Akiko |last15=Kristensen |first15=Reinhardt Møbjerg |last16=Wheeler |first16=Ward C. |last17=Martindale |first17=Mark Q. |last18=Giribet |first18=Gonzalo |display-authors=6 |title=Broad phylogenomic sampling improves resolution of the animal tree of life |journal=Nature |volume=452 |issue=7188 |pages=745–9 |date=April 2008 |pmid=18322464 |doi=10.1038/nature06614 |bibcode=2008Natur.452..745D |s2cid=4397099 }}</ref><ref name="Hejnol_2009">{{cite journal |last1=Hejnol |first1=Andreas |last2=Obst |first2=Matthias |last3=Stamatakis |first3=Alexandros |last4=Ott |first4=Michael |last5=Rouse |first5=Greg W. |last6=Edgecombe |first6=Gregory D. |last7=Martinez |first7=Pedro |last8=Baguñà |first8=Jaume |last9=Bailly |first9=Xavier |last10=Jondelius |first10=Ulf |last11=Wiens |first11=Matthias |last12=Müller |first12=Werner E. G. |last13=Seaver |first13=Elaine |last14=Wheeler |first14=Ward C. |last15=Martindale |first15=Mark Q. |last16=Giribet |first16=Gonzalo |last17=Dunn |first17=Casey W. |display-authors=6 |title=Assessing the root of bilaterian animals with scalable phylogenomic methods |journal=Proceedings. Biological Sciences |volume=276 |issue=1677 |pages=4261–70 |date=December 2009 |pmid=19759036 |pmc=2817096 |doi=10.1098/rspb.2009.0896 }}</ref><ref name="Ryan_2013">{{cite journal |last1=Ryan |first1=Joseph F. |last2=Pang |first2=Kevin |last3=Schnitzler |first3=Christine E. |last4=Nguyen |first4=Anh-Dao |last5=Moreland |first5=R. Travis |last6=Simmons |first6=David K. |last7=Koch |first7=Bernard J. |last8=Francis |first8=Warren R. |last9=Havlak |first9=Paul |author10=NISC Comparative Sequencing Program |last11=Smith |first11=Stephen A. |last12=Putnam |first12=Nicholas H. |last13=Haddock |first13=Steven H. D. |last14=Dunn |first14=Casey W. |last15=Wolfsberg |first15=Tyra G. |last16=Mullikin |first16=James C. |last17=Martindale |first17=Mark Q. |last18=Baxevanis |first18=Andreas D. |display-authors=6 |title=The genome of the ctenophore Mnemiopsis leidyi and its implications for cell type evolution |journal=Science |volume=342 |issue=6164 |page=1242592 |date=December 2013 |pmid=24337300 |pmc=3920664 |doi=10.1126/science.1242592 }}</ref><ref name="Moroz_2014">{{cite journal |last1=Moroz |first1=Leonid L. |last2=Kocot |first2=Kevin M. |last3=Citarella |first3=Mathew R. |last4=Dosung |first4=Sohn |last5=Norekian |first5=Tigran P. |last6=Povolotskaya |first6=Inna S. |last7=Grigorenko |first7=Anastasia P. |last8=Dailey |first8=Christopher |last9=Berezikov |first9=Eugene |last10=Buckley |first10=Katherine M. |last11=Ptitsyn |first11=Andrey |last12=Reshetov |first12=Denis |last13=Mukherjee |first13=Krishanu |last14=Moroz |first14=Tatiana P. |last15=Bobkova |first15=Yelena |last16=Yu |first16=Fahong |last17=Kapitonov |first17=Vladimir V. |last18=Jurka |first18=Jerzy |last19=Bobkov |first19=Yuri V. |last20=Swore |first20=Joshua J. |last21=Girardo |first21=David O. |last22=Fodor |first22=Alexander |last23=Gusev |first23=Fedor |last24=Sanford |first24=Rachel |last25=Bruders |first25=Rebecca |last26=Kittler |first26=Ellen |last27=Mills |first27=Claudia E. |last28=Rast |first28=Jonathan P. |last29=Derelle |first29=Romain |last30=Solovyev |first30=Victor V. |last31=Kondrashov |first31=Fyodor A. |last32=Swalla |first32=Billie J. |last33=Sweedler |first33=Jonathan V. |last34=Rogaev |first34=Evgeny I. |last35=Halanych |first35=Kenneth M. |last36=Kohn |first36=Andrea B. |display-authors=6 |title=The ctenophore genome and the evolutionary origins of neural systems |journal=Nature |volume=510 |issue=7503 |date=2014 |issn=0028-0836 |pmid=24847885 |pmc=4337882 |doi=10.1038/nature13400 |pages=109–114|bibcode=2014Natur.510..109M }}</ref> If this is correct, either modern comb jellies developed their complex structures independently of other Metazoa, or sponges' ancestors were more complex and all known sponges are drastically simplified forms. The study recommended further analyses using a wider range of sponges and other simple Metazoa such as [[Placozoa]].<ref name="Dunn_2008"/> |
||
However, reanalysis of the data showed that the computer algorithms used for analysis were misled by the presence of specific ctenophore genes that were markedly different from those of other species, leaving sponges as either the sister group to all other animals, or an ancestral paraphyletic grade.<ref>{{ |
However, reanalysis of the data showed that the computer algorithms used for analysis were misled by the presence of specific ctenophore genes that were markedly different from those of other species, leaving sponges as either the sister group to all other animals, or an ancestral paraphyletic grade.<ref>{{cite journal |last1=Pisani |first1=Davide |last2=Pett |first2=Walker |last3=Dohrmann |first3=Martin |last4=Feuda |first4=Roberto |last5=Rota-Stabelli |first5=Omar |last6=Philippe |first6=Hervé |last7=Lartillot |first7=Nicolas |last8=Wörheide |first8=Gert |title=Genomic data do not support comb jellies as the sister group to all other animals |journal=Proceedings of the National Academy of Sciences of the United States of America |volume=112 |issue=50 |pages=15402–15407 |date=December 2015 |pmid=26621703 |pmc=4687580 |doi=10.1073/pnas.1518127112 |doi-access=free |bibcode=2015PNAS..11215402P }}</ref><ref>{{Cite book |title=Spineless: the science of jellyfish and the art of growing a backbone |last= Berwald |first=Juli |publisher=Riverhead Books |year=2017|isbn=9780735211261}}{{page needed|date=October 2018}}</ref> 'Family trees' constructed using a combination of all available data – morphological, developmental and molecular – concluded that the sponges are in fact a monophyletic group, and with the [[cnidarian]]s form the sister group to the bilaterians.<ref name="Schierwater_2009">{{cite journal |last1=Schierwater |first1=Bernd |last2=Eitel |first2=Michael |last3=Jakob |first3=Wolfgang |last4=Osigus |first4=Hans-Jürgen |last5=Hadrys |first5=Heike |last6=Dellaporta |first6=Stephen L. |last7=Kolokotronis |first7=Sergios-Orestis |last8=DeSalle |first8=Rob |title=Concatenated analysis sheds light on early metazoan evolution and fuels a modern "urmetazoon" hypothesis |journal=PLOS Biology |volume=7 |issue=1 |pages=e20 |date=January 2009 |pmid=19175291 |pmc=2631068 |doi=10.1371/journal.pbio.1000020 |doi-access=free }}</ref><ref name="Kapli_2020">{{cite journal |last1= Kapli |first1=P. |last2=Telford |first2=M.J. |title=Topology-dependent asymmetry in systematic errors affects phylogenetic placement of Ctenophora and Xenacoelomorpha |journal=Science Advances |volume=6 |issue=50 |pages=eabc5162 |date=December 2020 |pmid=33310849 |pmc=7732190 |doi=10.1126/sciadv.abc5162 |doi-access=free |bibcode=2020SciA....6.5162K }}</ref> |
||
A very large and internally consistent alignment of 1,719 proteins at the metazoan scale, published in 2017, showed that (i) sponges – represented by Homoscleromorpha, Calcarea, Hexactinellida, and Demospongiae – are monophyletic, (ii) sponges are sister-group to all other multicellular animals, (iii) ctenophores emerge as the second-earliest branching animal lineage, and (iv) [[placozoans]] emerge as the third animal lineage, followed by [[Planulozoa|cnidarians sister-group to bilaterians]].<ref name="Simion_2017"/> |
A very large and internally consistent alignment of 1,719 proteins at the metazoan scale, published in 2017, showed that (i) sponges – represented by Homoscleromorpha, Calcarea, Hexactinellida, and Demospongiae – are monophyletic, (ii) sponges are sister-group to all other multicellular animals, (iii) ctenophores emerge as the second-earliest branching animal lineage, and (iv) [[placozoans]] emerge as the third animal lineage, followed by [[Planulozoa|cnidarians sister-group to bilaterians]].<ref name="Simion_2017">{{cite journal |last1=Simion |first1=Paul |last2=Philippe |first2=Hervé |last3=Baurain |first3=Denis |last4=Jager |first4=Muriel |last5=Richter |first5=Daniel J. |last6=Di Franco |first6=Arnaud |last7=Roure |first7=Béatrice |last8=Satoh |first8=Nori |last9=Quéinnec |first9=Éric |last10=Ereskovsky |first10=Alexander |last11=Lapébie |first11=Pascal |last12=Corre |first12=Erwan |last13=Delsuc |first13=Frédéric |last14=King |first14=Nicole |last15=Wörheide |first15=Gert |last16=Manuel |first16=Michaël |display-authors=6 |title=A Large and Consistent Phylogenomic Dataset Supports Sponges as the Sister Group to All Other Animals |journal=Current Biology |volume=27 |issue=7 |pages=958–967 |date=April 2017 |pmid=28318975 |doi=10.1016/j.cub.2017.02.031 |url=https://hal.archives-ouvertes.fr/hal-01681528/file/Simion_etal2017_CurrBiol_proofs.pdf |type=<!-- Submitted manuscript --> |doi-access=free |bibcode=2017CBio...27..958S |access-date=2018-11-04 |archive-date=2020-04-25 |archive-url=https://web.archive.org/web/20200425191351/https://hal.archives-ouvertes.fr/hal-01681528/file/Simion_etal2017_CurrBiol_proofs.pdf |url-status=live }}</ref> |
||
In March 2021, scientists from Dublin found additional evidence that sponges are the sister group to all other animals |
In March 2021, scientists from Dublin found additional evidence that sponges are the sister group to all other animals,<ref>{{cite journal |last1= Redmond |first1=A.K. |last2=McLysaght |first2=A. |title=Evidence for sponges as sister to all other animals from partitioned phylogenomics with mixture models and recoding |journal=Nature Communications |volume=12 |issue=1 |pages=1783 |date=March 2021 |pmid=33741994 |pmc=7979703 |doi=10.1038/s41467-021-22074-7 |doi-access=free |bibcode=2021NatCo..12.1783R }}</ref> while in May 2023, Schultz et al. found patterns of irreversible change in genome synteny that provide strong evidence that [[ctenophora|ctenophores]] are the sister group to all other animals instead.<ref>{{cite journal |last1=Schultz |first1=Darrin T. |last2=Haddock |first2=Steven H. D. |last3=Bredeson |first3=Jessen V. |last4=Green |first4=Richard E. |last5=Simakov |first5=Oleg |last6=Rokhsar |first6=Daniel S. |title=Ancient gene linkages support ctenophores as sister to other animals |journal=Nature |volume=618 |issue=7963 |pages=110–117 |date=June 2023 |pmid=37198475 |pmc=10232365 |doi=10.1038/s41586-023-05936-6 |bibcode=2023Natur.618..110S }}</ref> |
||
== Notable spongiologists == |
== Notable spongiologists == |
||
| Line 502: | Line 520: | ||
== Use == |
== Use == |
||
[[File:2008.09-331-196ap Sponge gourd,pd Spice Bazaar@Istanbul,TR mon29sep2008-1315h.jpg|thumb|Sponges made of [[Luffa aegyptiaca|sponge gourd]] for sale alongside sponges of animal origin ([[Spice Bazaar, Istanbul|Spice Bazaar at Istanbul]], Turkey)]] |
[[File:2008.09-331-196ap Sponge gourd,pd Spice Bazaar@Istanbul,TR mon29sep2008-1315h.jpg|thumb|Sponges made of [[Luffa aegyptiaca|sponge gourd]] for sale alongside sponges of animal origin ([[Spice Bazaar, Istanbul|Spice Bazaar at Istanbul]], Turkey)]] |
||
=== By dolphins === |
=== By dolphins === |
||
A report in 1997 described use of sponges [[Tool use by non-human animals|as a tool]] by [[bottlenose dolphins]] in [[Shark Bay]] in Western Australia. A dolphin will attach a marine sponge to its [[rostrum (anatomy)|rostrum]], which is presumably then used to protect it when searching for food in the sandy [[sea floor|sea bottom]].<ref name="Smolker 1997">{{cite journal | vauthors = Smolker RA, Richards AF, Connor RC, Mann J, Berggren P |title=Sponge-carrying by Indian Ocean bottlenose dolphins: Possible tool-use by a delphinid|journal=Ethology|year=1997|volume=103| pages=454–465|doi=10.1111/j.1439-0310.1997.tb00160.x|issue=6 |hdl=2027.42/71936|hdl-access=free}}</ref> The behavior, known as ''sponging'', has only been observed in this bay and is almost exclusively shown by females.<!-- This is the only known case of tool use in [[marine mammal]]s outside of [[sea otter]]s.--> A study in 2005 concluded that mothers teach the behavior to their daughters and that all the sponge users are closely related, suggesting that it is a fairly recent innovation.<ref name="Krutzen 2005">{{cite journal | vauthors = Krützen M, Mann J, Heithaus MR, Connor RC, Bejder L, Sherwin WB | title = Cultural transmission of tool use in bottlenose dolphins | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 102 | issue = 25 | pages = 8939–43 | date = June 2005 | pmid = 15947077 | pmc = 1157020 | doi = 10.1073/pnas.0500232102 | bibcode = 2005PNAS..102.8939K | doi-access = free }}</ref> |
|||
A report in 1997 described use of sponges [[Tool use by non-human animals|as a tool]] by [[bottlenose dolphins]] in [[Shark Bay]] in Western Australia. A dolphin will attach a marine sponge to its [[rostrum (anatomy)|rostrum]], which is presumably then used to protect it when searching for food in the sandy [[sea floor|sea bottom]].<ref name="Smolker 1997">{{cite journal |last1=Smolker |first1=Rachel |last2=Richards |first2=Andrew |last3=Connor |first3=Richard |last4=Mann |first4=Janet |last5=Berggren |first5=Per |title=Sponge-carrying by Indian Ocean bottlenose dolphins: Possible tool-use by a delphinid|journal=Ethology|year=1997|volume=103|pages=454–465|doi=10.1111/j.1439-0310.1997.tb00160.x|issue=6 |hdl=2027.42/71936|hdl-access=free}}</ref> The behavior, known as ''sponging'', has only been observed in this bay and is almost exclusively shown by females.<!-- This is the only known case of tool use in [[marine mammal]]s outside of [[sea otter]]s.--> A study in 2005 concluded that mothers teach the behavior to their daughters and that all the sponge users are closely related, suggesting that it is a fairly recent innovation.<ref name="Krutzen_2005">{{cite journal |last1=Krützen |first1=Michael |last2=Mann |first2=Janet |last3=Heithaus |first3=Michael R. |last4=Connor |first4=Richard C. |last5=Bejder |first5=Lars |last6=Sherwin |first6=William B. |title=Cultural transmission of tool use in bottlenose dolphins |journal=Proceedings of the National Academy of Sciences of the United States of America |volume=102 |issue=25 |pages=8939–43 |date=June 2005 |pmid=15947077 |pmc=1157020 |doi=10.1073/pnas.0500232102 |bibcode=2005PNAS..102.8939K |doi-access=free }}</ref> |
|||
=== By humans === |
=== By humans === |
||
{{Main|Sea sponge aquaculture|Sponge diving}} |
{{Main|Sea sponge aquaculture|Sponge diving}} |
||
[[File:SpongesTarponSprings.jpg|thumb|Natural sponges in [[Tarpon Springs]], [[Florida]]]] |
[[File:SpongesTarponSprings.jpg|thumb|Natural sponges in [[Tarpon Springs]], [[Florida]]]] |
||
[[File:Sponges.JPG|right|thumb|Display of natural sponges for sale on [[Kalymnos]] in [[Greece]]]] |
[[File:Sponges.JPG|right|thumb|Display of natural sponges for sale on [[Kalymnos]] in [[Greece]]]] |
||
==== Skeleton ==== |
==== Skeleton ==== |
||
{{Main|Sponge (material)}} |
{{Main|Sponge (material)}} |
||
The [[calcium carbonate]] or [[silica]] [[Sponge spicule|spicules]] of most sponge [[genus|genera]] make them too rough for most uses, but two genera, ''[[Hippospongia]]'' and ''[[Spongia]]'', have soft, entirely fibrous skeletons.{{sfn|Bergquist|1978|p=88}} Early Europeans used soft sponges for many purposes, including padding for helmets, portable drinking utensils and municipal water filters. Until the invention of synthetic sponges, they were used as cleaning tools, applicators for paints and [[ceramic glaze]]s and discreet [[contraceptive]]s. However, by the mid-20th century, overfishing brought both the animals and the industry close to [[extinction]].<ref>{{cite book| vauthors = McClenachan L |chapter=Social conflict, Over-fishing and Disease in the Florida Sponge Fishery, 1849–1939|pages=25–27|title=Oceans Past: Management Insights from the History of Marine Animal Populations| veditors = Starkey DJ, Holm P, Barnard M |publisher=[[Earthscan]]|year=2008|isbn=978-1-84407-527-0|chapter-url={{google books |plainurl=y |id=cGEeEfFegvEC|page=26}}}}</ref> |
|||
The [[calcium carbonate]] or [[silica]] [[Sponge spicule|spicules]] of most sponge [[genus|genera]] make them too rough for most uses, but two genera, ''[[Hippospongia]]'' and ''[[Spongia]]'', have soft, entirely fibrous skeletons.<ref name="Bergquist_1978"/>{{rp|88}} Early Europeans used soft sponges for many purposes, including padding for helmets, portable drinking utensils and municipal water filters. Until the invention of synthetic sponges, they were used as cleaning tools, applicators for paints and [[ceramic glaze]]s and discreet [[contraceptive]]s. However, by the mid-20th century, overfishing brought both the animals and the industry close to [[extinction]].<ref>{{cite book |last=McClenachan |first=L. |chapter=Social conflict, Over-fishing and Disease in the Florida Sponge Fishery, 1849–1939 |pages=25–27 |title=Oceans Past: Management Insights from the History of Marine Animal Populations |editor1=Starkey, D.J. |editor2=Holm, P. |editor3=Barnard, M. |publisher=[[Earthscan]] |year=2008 |isbn=978-1-84407-527-0 |chapter-url={{google books |plainurl=y |id=cGEeEfFegvEC|page=26}}}}</ref> |
|||
Many objects with sponge-like textures are now made of substances not derived from poriferans. Synthetic sponges include personal and household [[Sponge (material)|cleaning tools]], [[breast implant]]s,<ref>{{cite book| vauthors = Jacobson N |title=Cleavage| publisher=Rutgers University Press|isbn=978-0-8135-2715-4|url={{google books |plainurl=y |id=3ZIw_3Px4AEC|page=62}}|year=2000|page=62}}</ref> and [[contraceptive sponge]]s.<ref name="CBAS">{{Cite journal|title=Sponges |journal=[[Cervical Barrier Advancement Society]] |year=2004 |url=http://www.cervicalbarriers.org/information/sponges.cfm |access-date=2006-09-17 |archive-date=January 14, 2009 |url-status=dead |archive-url=https://web.archive.org/web/20090114062634/http://www.cervicalbarriers.org/information/sponges.cfm }}</ref> Typical materials used are [[cellulose]] foam, [[polyurethane]] foam, and less frequently, [[silicone]] foam. |
|||
Many objects with sponge-like textures are now made of substances not derived from poriferans. Synthetic sponges include personal and household [[Sponge (material)|cleaning tools]], [[breast implant]]s,<ref>{{cite book |last=Jacobson |first=N. |title=Cleavage |publisher=Rutgers University Press|isbn=978-0-8135-2715-4|url={{google books |plainurl=y |id=3ZIw_3Px4AEC|page=62}}|year=2000|page=62}}</ref> and [[contraceptive sponge]]s.<ref name="CBAS">{{Cite journal |title=Sponges |journal=[[Cervical Barrier Advancement Society]] |year=2004 |url=http://www.cervicalbarriers.org/information/sponges.cfm |access-date=2006-09-17 |archive-date=January 14, 2009 |url-status=dead |archive-url=https://web.archive.org/web/20090114062634/http://www.cervicalbarriers.org/information/sponges.cfm }}</ref> Typical materials used are [[cellulose]] foam, [[polyurethane]] foam, and less frequently, [[silicone]] foam. |
|||
The [[luffa]] "sponge", also spelled ''loofah'', which is commonly sold for use in the kitchen or the shower, is not derived from an animal but mainly from the fibrous "skeleton" of the [[Luffa aegyptiaca|sponge gourd]] (''Luffa aegyptiaca'', [[Cucurbitaceae]]).<ref>{{cite journal | vauthors = Porterfield WM | title = Loofah — The sponge gourd | journal = [[Economic Botany]] | volume = 9 | issue = 3 | year = 1955 | pages = 211–223 | doi = 10.1007/BF02859814 | s2cid = 27313678 }}</ref> |
|||
The [[luffa]] "sponge", also spelled ''loofah'', which is commonly sold for use in the kitchen or the shower, is not derived from an animal but mainly from the fibrous "skeleton" of the [[Luffa aegyptiaca|sponge gourd]] (''Luffa aegyptiaca'', [[Cucurbitaceae]]).<ref>{{cite journal |last=Porterfield |first=W.M. |title=Loofah — The sponge gourd |journal=[[Economic Botany]] |volume=9 |issue=3 |year=1955 |pages=211–223 |doi=10.1007/BF02859814 |bibcode=1955EcBot...9..211P |s2cid=27313678 }}</ref> |
|||
==== Antibiotic compounds ==== |
==== Antibiotic compounds ==== |
||
Sponges have [[medicine|medicinal]] potential due to the presence in sponges themselves or their microbial [[symbiosis|symbionts]] of chemicals that may be used to control [[virus]]es, [[bacteria]], [[tumor]]s and fungi.<ref>{{cite book | vauthors = Imhoff JF, Stöhr R | chapter = Sponge-Associated Bacteria | pages = 43–44 | veditors = Müller WE | title = Sponges (Porifera): Porifera | publisher = [[Springer Science+Business Media|Springer]] | year = 2003 | isbn = 978-3-540-00968-9 }}</ref><ref name="pmid8297426">{{cite journal | vauthors = Teeyapant R, Woerdenbag HJ, Kreis P, Hacker J, Wray V, Witte L, Proksch P | title = Antibiotic and cytotoxic activity of brominated compounds from the marine sponge Verongia aerophoba | journal = Zeitschrift für Naturforschung C | volume = 48 | issue = 11–12 | pages = 939–45 | date = 1993 | pmid = 8297426 | doi = 10.1515/znc-1993-11-1218 | s2cid = 1593418 | doi-access = free }}</ref> |
|||
Sponges have [[medicine|medicinal]] potential due to the presence in sponges themselves or their microbial [[symbiosis|symbionts]] of chemicals that may be used to control [[virus]]es, [[bacteria]], [[tumor]]s and fungi.<ref>{{cite book |last1=Imhoff |first1=J.F. |last2=Stöhr |first2=R. |chapter=Sponge-Associated Bacteria |pages=43–44 |editor= Müller, W.E. |title=Sponges (Porifera): Porifera |publisher=[[Springer Science+Business Media|Springer]] |year=2003 |isbn=978-3-540-00968-9 }}</ref><ref name="pmid8297426">{{cite journal |last1=Teeyapant |first1=R. |last2=Woerdenbag |first2=H. J. |last3=Kreis |first3=P. |last4=Hacker |first4=J. |last5=Wray |first5=V. |last6=Witte |first6=L. |last7=Proksch |first7=P. |title=Antibiotic and cytotoxic activity of brominated compounds from the marine sponge Verongia aerophoba |journal=Zeitschrift für Naturforschung C |volume=48 |issue=11–12 |pages=939–45 |date=1993 |pmid=8297426 |doi=10.1515/znc-1993-11-1218 |s2cid=1593418 |doi-access=free }}</ref> |
|||
==== Other biologically active compounds ==== |
==== Other biologically active compounds ==== |
||
{{Main|Sponge isolates}} |
{{Main|Sponge isolates}} |
||
[[File:Halichondria and Eribulin.jpg|thumb|upright|''Halichondria'' produces the [[eribulin]] precursor [[halichondrin B]]]] |
[[File:Halichondria and Eribulin.jpg|thumb|upright|''Halichondria'' produces the [[eribulin]] precursor [[halichondrin B]]]] |
||
Lacking any protective shell or means of escape, sponges have evolved to synthesize a variety of unusual compounds. One such class is the oxidized fatty acid derivatives called [[oxylipin]]s. Members of this family have been found to have anti-cancer, anti-bacterial and anti-fungal properties. One example isolated from the Okinawan ''plakortis'' sponges, [[plakoridine A]], has shown potential as a cytotoxin to murine lymphoma cells.<ref>{{cite journal | vauthors = Takeuchi S, Ishibashi M, Kobayashi J, Plakoridine A |title=Plakoridine A, a new tyramine-containing pyrrolidine alkaloid from the ''Okinawan marine'' sponge Plakortis sp |journal=Journal of Organic Chemistry |year=1994 |volume=59 |issue=13 |pages=3712–3713 |doi=10.1021/jo00092a039 }}</ref><ref>{{cite journal| vauthors = Etchells LL, Sardarian A, Whitehead RC |title=A synthetic approach to the plakoridines modeled on a biogenetic theory |journal=[[Tetrahedron Letters]]|date=18 April 2005|volume=46|issue=16|pages=2803–2807|doi=10.1016/j.tetlet.2005.02.124 }}</ref> |
|||
Lacking any protective shell or means of escape, sponges have evolved to synthesize a variety of unusual compounds. One such class is the oxidized fatty acid derivatives called [[oxylipin]]s. Members of this family have been found to have anti-cancer, anti-bacterial and anti-fungal properties. One example isolated from the Okinawan ''plakortis'' sponges, [[plakoridine A]], has shown potential as a cytotoxin to murine lymphoma cells.<ref>{{cite journal |last1=Takeuchi |first1=Shinji |last2=Ishibashi |first2=Masami |last3=Kobayashi |first3=Junichi |title=Plakoridine A, a new tyramine-containing pyrrolidine alkaloid from the ''Okinawan marine'' sponge Plakortis sp |journal=Journal of Organic Chemistry |year=1994 |volume=59 |issue=13 |pages=3712–3713 |doi=10.1021/jo00092a039 }}</ref><ref>{{cite journal |last1=Etchells |first1=Laura L. |last2=Sardarian |first2=Ali |last3=Whitehead |first3=Roger C. |title=A synthetic approach to the plakoridines modeled on a biogenetic theory |journal=[[Tetrahedron Letters]]|date=18 April 2005|volume=46|issue=16|pages=2803–2807|doi=10.1016/j.tetlet.2005.02.124 }}</ref> |
|||
{{Clear}} |
{{Clear}} |
||
== See also == |
== See also == |
||
{{Portal bar|Marine Life|Animals|Earth sciences}} |
|||
* [[Lists of sponges]] |
* [[Lists of sponges]] |
||
* [[Sponge Reef Project]] |
* [[Sponge Reef Project]] |
||
* [[SpongeBob SquarePants]] |
|||
* [[3-Alkylpyridinium]], compounds found in marine [[Haplosclerida]] sponges |
* [[3-Alkylpyridinium]], compounds found in marine [[Haplosclerida]] sponges |
||
== References == |
== References == |
||
<!-- Molecular Phylogenetics and Evolution 47 (2008) 433–438 --> |
|||
{{Reflist|30em|refs= |
|||
{{Reflist}} |
|||
<ref name=GageTyler91-93>{{Harvard citation no brackets|Gage|Tyler|1996|pages=91–93}}</ref> |
|||
== Further reading == |
|||
<ref name="RuppertBarnes2004Porifera">{{Harvard citation no brackets|Ruppert|Fox|Barnes|2004|pages=76–97}}</ref> |
|||
}} |
|||
== Bibliography == |
|||
{{refbegin|30em}} |
{{refbegin|30em}} |
||
* {{ |
* {{cite book |author1=Hickman, C. |author2=Roberts, L. |author3=Larson, A. |year=2003 |title=Animal Diversity |edition=3rd |publisher=McGraw-Hill |location=New York |isbn=978-0-07-234903-0 |url-access=registration |url=https://archive.org/details/animaldiversity0003hick }} |
||
* {{cite book |author=Ereskovsky, A.V. |title=The Comparative Embryology of Sponges|year=2010|location=Russia|publisher=Springer Science+Business Media|isbn=978-90-481-8575-7}} |
|||
* {{Cite book |vauthors=Hickman C, Roberts L, Larson A |year=2003 |title=Animal Diversity |edition=3rd |publisher=McGraw-Hill |location=New York |isbn=978-0-07-234903-0 |url-access=registration |url=https://archive.org/details/animaldiversity0003hick }} |
|||
* {{cite journal |author=Wörheide, G. |title=A hypercalcified sponge with soft relatives: Vaceletia is a keratose demosponge |journal=Molecular Phylogenetics and Evolution |volume=47 |issue=1 |pages=433–8 |date=April 2008 |pmid=18321733 |doi=10.1016/j.ympev.2008.01.021 |bibcode=2008MolPE..47..433W }} |
|||
* {{cite book|last=Ereskovsky|first=Alexander V. | name-list-style = vanc |title=The Comparative Embryology of Sponges|year=2010|location=Russia|publisher=Springer Science+Business Media|isbn=978-90-481-8575-7}} |
|||
* {{cite book |last1=Piper |first1=Ross |name-list-style=vanc |author-link1=Ross Piper |year=2007 |title=Extraordinary Animals: An Encyclopedia of Curious and Unusual Animals |publisher=[[Greenwood Publishing Group]] |isbn=978-0-313-33922-6 |url-access=registration |url=https://archive.org/details/extraordinaryani0000pipe }} |
|||
* {{cite book|last1=Ruppert|first1=Edward E.|last2=Fox|first2=Richard S.|last3=Barnes|first3=Robert D.|name-list-style=vanc|title=Invertebrate Zoology|publisher=Brooks / [[COLE Publishing]]|edition=7|isbn=978-0-03-025982-1|year=2004|url-access=registration|url=https://archive.org/details/isbn_9780030259821}} |
|||
* {{cite book|title= Coral Reefs: Cities Under The Seas|last=Murphy|first=Richard C.| name-list-style = vanc |year=2002|isbn=978-0-87850-138-0|publisher=[[The Darwin Press]], Inc.}} |
|||
* {{cite book|last1=Gage|first1=John D.|last2=Tyler|first2=Paul A.| name-list-style = vanc |title=Deep-sea Biology: A Natural History of Organisms at the Deep-Sea Floor|publisher=[[Cambridge University Press]]|year=1996|isbn=978-0-521-33665-9|url={{google books |plainurl=y |id=kHZO5igKhsAC}}}} |
|||
* {{cite journal|doi=10.1007/s00435-004-0100-0| vauthors = Vacelet J, Duport E |title=Prey capture and digestion in the carnivorous sponge ''Asbestopluma hypogea'' (Porifera: Demospongiae)|year=2004|journal=[[Zoomorphology]]|volume=123|issue=4|pages=179–190| s2cid = 24484610 }} |
|||
{{refend}} |
{{refend}} |
||
== External links == |
== External links == |
||
{{Commons category|Porifera}} |
{{Commons category|Porifera}} |
||
{{Wikispecies|Porifera}} |
{{Wikispecies|Porifera}} |
||
{{Wikibooks|Dichotomous Key|Porifera}} |
{{Wikibooks|Dichotomous Key|Porifera}} |
||
{{EB1911 poster|Sponges}} |
|||
* [http://www.biology.ualberta.ca/courses.hp/zool250/animations/Porifera.swf Water flow and feeding in the phylum Porifera (sponges)] – [[Adobe Flash|Flash]] animations of sponge body structures, water flow and feeding |
* [http://www.biology.ualberta.ca/courses.hp/zool250/animations/Porifera.swf Water flow and feeding in the phylum Porifera (sponges)] – [[Adobe Flash|Flash]] animations of sponge body structures, water flow and feeding |
||
* [http://www.spongepage.info/ Carsten's Spongepage], Information on the ecology and the biotechnological potential of sponges and their associated bacteria. |
* [http://www.spongepage.info/ Carsten's Spongepage], Information on the ecology and the biotechnological potential of sponges and their associated bacteria. |
||
| Line 571: | Line 590: | ||
* [https://web.archive.org/web/20121001131301/http://www.qm.qld.gov.au/Collections/Biodiversity+and+Geosciences/Biodiversity+Collections/Sessile+marine+invertebrates Queensland Museum Sessile marine invertebrates collections] |
* [https://web.archive.org/web/20121001131301/http://www.qm.qld.gov.au/Collections/Biodiversity+and+Geosciences/Biodiversity+Collections/Sessile+marine+invertebrates Queensland Museum Sessile marine invertebrates collections] |
||
* [https://web.archive.org/web/20121001131444/http://www.qm.qld.gov.au/Research/Biodiversity/Areas+of+research/Sessile+marine+invertebrates Queensland Museum Sessile marine invertebrates research] |
* [https://web.archive.org/web/20121001131444/http://www.qm.qld.gov.au/Research/Biodiversity/Areas+of+research/Sessile+marine+invertebrates Queensland Museum Sessile marine invertebrates research] |
||
* [http://www.habitas.org.uk/marinelife/sponge_guide/ Sponge Guide for Britain and Ireland], Bernard Picton, Christine Morrow & Rob van Soest |
* [http://www.habitas.org.uk/marinelife/sponge_guide/ Sponge Guide for Britain and Ireland] {{Webarchive|url=https://web.archive.org/web/20081208120849/http://www.habitas.org.uk/marinelife/sponge_guide/ |date=2008-12-08 }}, Bernard Picton, Christine Morrow & Rob van Soest |
||
* [http://www.marinespecies.org/porifera/ World Porifera database], the world list of extant sponges, includes a searchable database. |
* [http://www.marinespecies.org/porifera/ World Porifera database], the world list of extant sponges, includes a searchable database. |
||
* [http://www.fao.org/3/AC286E/AC286E00.htm#TOC Sponges: World production and markets] // Food and Agriculture Organisation |
* [http://www.fao.org/3/AC286E/AC286E00.htm#TOC Sponges: World production and markets] // Food and Agriculture Organisation |
||
| Line 578: | Line 597: | ||
{{Animalia}} |
{{Animalia}} |
||
{{Life on Earth}} |
{{Life on Earth}} |
||
{{Portal bar|Marine Life|Animals|Earth sciences}} |
|||
{{Taxonbar|from=Q18960}} |
{{Taxonbar|from=Q18960}} |
||
{{Authority control}} |
{{Authority control}} |
||
[[Category:Sponges| |
[[Category:Sponges|Sponges]] |
||
[[Category:Aquatic animals]] |
[[Category:Aquatic animals]] |
||
<!-- [[Category:Animal phyla]] |
<!-- [[Category:Animal phyla]] |
||
Latest revision as of 21:15, 29 October 2024
| Sponges | |
|---|---|

| |
| A stove-pipe sponge | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Porifera Grant, 1836 |
| Classes | |
| Synonyms | |
Sponges or sea sponges are members of the metazoan phylum Porifera[4] (/pəˈrɪfərəˌ pɔː-/ pər-IF-ər-ə, por-; meaning 'pore bearer'),[5] a basal animal clade and a sister taxon of the diploblasts.[6] They are sessile filter feeders that are bound to the seabed, and are one of the most ancient members of macrobenthos, with many historical species being important reef-building organisms.
Sponges are multicellular organisms consisting of jelly-like mesohyl sandwiched between two thin layers of cells, and usually have tube-like bodies full of pores and channels that allow water to circulate through them. They have unspecialized cells that can transform into other types and that often migrate between the main cell layers and the mesohyl in the process. They do not have complex nervous,[7] digestive or circulatory systems. Instead, most rely on maintaining a constant water flow through their bodies to obtain food and oxygen and to remove wastes, usually via flagella movements of the so-called "collar cells".
Believed to be some of the most basal animals alive today, sponges were possibly the first outgroup to branch off the evolutionary tree from the last common ancestor of all animals,[6] with fossil evidence of primitive sponges such as Otavia from as early as the Tonian period (around 800 Mya). The branch of zoology that studies sponges is known as spongiology.[8]
Etymology
The term sponge derives from the Ancient Greek word σπόγγος spóngos.[9] The scientific name Porifera is a neuter plural of the Modern Latin term porifer, which comes from the roots porus meaning "pore, opening", and -fer meaning "bearing or carrying".
Overview

Sponges are similar to other animals in that they are multicellular, heterotrophic, lack cell walls and produce sperm cells. Unlike other animals, they lack true tissues[10] and organs.[11] Some of them are radially symmetrical, but most are asymmetrical. The shapes of their bodies are adapted for maximal efficiency of water flow through the central cavity, where the water deposits nutrients and then leaves through a hole called the osculum. The single-celled choanoflagellates resemble the choanocyte cells of sponges which are used to drive their water flow systems and capture most of their food. This along with phylogenetic studies of ribosomal molecules have been used as morphological evidence to suggest sponges are the sister group to the rest of animals.[12] A great majority are marine (salt-water) species, ranging in habitat from tidal zones to depths exceeding 8,800 m (5.5 mi), though there are freshwater species. All adult sponges are sessile, meaning that they attach to an underwater surface and remain fixed in place (i.e., do not travel). While in their larval stage of life, they are motile.
Many sponges have internal skeletons of spicules (skeletal-like fragments of calcium carbonate or silicon dioxide), and/or spongin (a modified type of collagen protein).[10] An internal gelatinous matrix called mesohyl functions as an endoskeleton, and it is the only skeleton in soft sponges that encrust such hard surfaces as rocks. More commonly, the mesohyl is stiffened by mineral spicules, by spongin fibers, or both. 90% of all known sponge species that have the widest range of habitats including all freshwater ones are demosponges that use spongin; many species have silica spicules, whereas some species have calcium carbonate exoskeletons. Calcareous sponges have calcium carbonate spicules and, in some species, calcium carbonate exoskeletons, are restricted to relatively shallow marine waters where production of calcium carbonate is easiest.[13]: 179 The fragile glass sponges, with "scaffolding" of silica spicules, are restricted to polar regions and the ocean depths where predators are rare. Fossils of all of these types have been found in rocks dated from 580 million years ago. In addition Archaeocyathids, whose fossils are common in rocks from 530 to 490 million years ago, are now regarded as a type of sponge.
Although most of the approximately 5,000–10,000 known species of sponges feed on bacteria and other microscopic food in the water, some host photosynthesizing microorganisms as endosymbionts, and these alliances often produce more food and oxygen than they consume. A few species of sponges that live in food-poor environments have evolved as carnivores that prey mainly on small crustaceans.[14]
Most sponges reproduce sexually, but they can also reproduce asexually. Sexually reproducing species release sperm cells into the water to fertilize ova released or retained by its mate or "mother"; the fertilized eggs develop into larvae which swim off in search of places to settle.[13]: 183–185 Sponges are known for regenerating from fragments that are broken off, although this only works if the fragments include the right types of cells. Some species reproduce by budding. When environmental conditions become less hospitable to the sponges, for example as temperatures drop, many freshwater species and a few marine ones produce gemmules, "survival pods" of unspecialized cells that remain dormant until conditions improve; they then either form completely new sponges or recolonize the skeletons of their parents.[13]: 120–127

The few species of demosponge that have entirely soft fibrous skeletons with no hard elements have been used by humans over thousands of years for several purposes, including as padding and as cleaning tools. By the 1950s, though, these had been overfished so heavily that the industry almost collapsed, and most sponge-like materials are now synthetic. Sponges and their microscopic endosymbionts are now being researched as possible sources of medicines for treating a wide range of diseases. Dolphins have been observed using sponges as tools while foraging.[16]
Distinguishing features
Sponges constitute the phylum Porifera, and have been defined as sessile metazoans (multicelled immobile animals) that have water intake and outlet openings connected by chambers lined with choanocytes, cells with whip-like flagella.[13]: 29 However, a few carnivorous sponges have lost these water flow systems and the choanocytes.[13]: 39 [17] All known living sponges can remold their bodies, as most types of their cells can move within their bodies and a few can change from one type to another.[17][18]
Even if a few sponges are able to produce mucus – which acts as a microbial barrier in all other animals – no sponge with the ability to secrete a functional mucus layer has been recorded. Without such a mucus layer their living tissue is covered by a layer of microbial symbionts, which can contribute up to 40–50% of the sponge wet mass. This inability to prevent microbes from penetrating their porous tissue could be a major reason why they have never evolved a more complex anatomy.[19]
Like cnidarians (jellyfish, etc.) and ctenophores (comb jellies), and unlike all other known metazoans, sponges' bodies consist of a non-living jelly-like mass (mesohyl) sandwiched between two main layers of cells.[20][21] Cnidarians and ctenophores have simple nervous systems, and their cell layers are bound by internal connections and by being mounted on a basement membrane (thin fibrous mat, also known as "basal lamina").[21] Sponges do not have a nervous system similar to that of vertebrates but may have one that is quite different.[7] Their middle jelly-like layers have large and varied populations of cells, and some types of cells in their outer layers may move into the middle layer and change their functions.[18]
| Sponges[18][20] | Cnidarians and ctenophores[21] | |
|---|---|---|
| Nervous system | No/Yes | Yes, simple |
| Cells in each layer bound together | No, except that Homoscleromorpha have basement membranes.[22] | Yes: inter-cell connections; basement membranes |
| Number of cells in middle "jelly" layer | Many | Few |
| Cells in outer layers can move inwards and change functions | Yes | No |
Basic structure
Cell types
A sponge's body is hollow and is held in shape by the mesohyl, a jelly-like substance made mainly of collagen and reinforced by a dense network of fibers also made of collagen. 18 distinct cell types have been identified.[24] The inner surface is covered with choanocytes, cells with cylindrical or conical collars surrounding one flagellum per choanocyte. The wave-like motion of the whip-like flagella drives water through the sponge's body. All sponges have ostia, channels leading to the interior through the mesohyl, and in most sponges these are controlled by tube-like porocytes that form closable inlet valves. Pinacocytes, plate-like cells, form a single-layered external skin over all other parts of the mesohyl that are not covered by choanocytes, and the pinacocytes also digest food particles that are too large to enter the ostia,[18][20] while those at the base of the animal are responsible for anchoring it.[20]
Other types of cells live and move within the mesohyl:[18][20]
- Lophocytes are amoeba-like cells that move slowly through the mesohyl and secrete collagen fibres.
- Collencytes are another type of collagen-producing cell.
- Rhabdiferous cells secrete polysaccharides that also form part of the mesohyl.
- Oocytes and spermatocytes are reproductive cells.
- Sclerocytes secrete the mineralized spicules ("little spines") that form the skeletons of many sponges and in some species provide some defense against predators.
- In addition to or instead of sclerocytes, demosponges have spongocytes that secrete a form of collagen that polymerizes into spongin, a thick fibrous material that stiffens the mesohyl.
- Myocytes ("muscle cells") conduct signals and cause parts of the animal to contract.
- "Grey cells" act as sponges' equivalent of an immune system.
- Archaeocytes (or amoebocytes) are amoeba-like cells that are totipotent, in other words, each is capable of transformation into any other type of cell. They also have important roles in feeding and in clearing debris that block the ostia.
Many larval sponges possess neuron-less eyes that are based on cryptochromes. They mediate phototaxic behavior.[25]
Glass sponges present a distinctive variation on this basic plan. Their spicules, which are made of silica, form a scaffolding-like framework between whose rods the living tissue is suspended like a cobweb that contains most of the cell types.[18] This tissue is a syncytium that in some ways behaves like many cells that share a single external membrane, and in others like a single cell with multiple nuclei.
Water flow and body structures
Most sponges work rather like chimneys: they take in water at the bottom and eject it from the osculum at the top. Since ambient currents are faster at the top, the suction effect that they produce by Bernoulli's principle does some of the work for free. Sponges can control the water flow by various combinations of wholly or partially closing the osculum and ostia (the intake pores) and varying the beat of the flagella, and may shut it down if there is a lot of sand or silt in the water.[18]
Although the layers of pinacocytes and choanocytes resemble the epithelia of more complex animals, they are not bound tightly by cell-to-cell connections or a basal lamina (thin fibrous sheet underneath). The flexibility of these layers and re-modeling of the mesohyl by lophocytes allow the animals to adjust their shapes throughout their lives to take maximum advantage of local water currents.[18]: 83
The simplest body structure in sponges is a tube or vase shape known as "asconoid", but this severely limits the size of the animal. The body structure is characterized by a stalk-like spongocoel surrounded by a single layer of choanocytes. If it is simply scaled up, the ratio of its volume to surface area increases, because surface increases as the square of length or width while volume increases proportionally to the cube. The amount of tissue that needs food and oxygen is determined by the volume, but the pumping capacity that supplies food and oxygen depends on the area covered by choanocytes. Asconoid sponges seldom exceed 1 mm (0.039 in) in diameter.[18]

Some sponges overcome this limitation by adopting the "syconoid" structure, in which the body wall is pleated. The inner pockets of the pleats are lined with choanocytes, which connect to the outer pockets of the pleats by ostia. This increase in the number of choanocytes and hence in pumping capacity enables syconoid sponges to grow up to a few centimeters in diameter.
The "leuconoid" pattern boosts pumping capacity further by filling the interior almost completely with mesohyl that contains a network of chambers lined with choanocytes and connected to each other and to the water intakes and outlet by tubes. Leuconid sponges grow to over 1 m (3.3 ft) in diameter, and the fact that growth in any direction increases the number of choanocyte chambers enables them to take a wider range of forms, for example, "encrusting" sponges whose shapes follow those of the surfaces to which they attach. All freshwater and most shallow-water marine sponges have leuconid bodies. The networks of water passages in glass sponges are similar to the leuconid structure.[18]
In all three types of structure the cross-section area of the choanocyte-lined regions is much greater than that of the intake and outlet channels. This makes the flow slower near the choanocytes and thus makes it easier for them to trap food particles.[18] For example, in Leuconia, a small leuconoid sponge about 10 centimetres (3.9 in) tall and 1 centimetre (0.39 in) in diameter, water enters each of more than 80,000 intake canals at 6 cm per minute. However, because Leuconia has more than 2 million flagellated chambers whose combined diameter is much greater than that of the canals, water flow through chambers slows to 3.6 cm per hour, making it easy for choanocytes to capture food. All the water is expelled through a single osculum at about 8.5 cm per second, fast enough to carry waste products some distance away.[27]
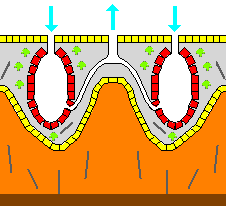
- Mesohyl
- Spicules
- Seabed / rock
- Water flow
Skeleton
In zoology a skeleton is any fairly rigid structure of an animal, irrespective of whether it has joints and irrespective of whether it is biomineralized. The mesohyl functions as an endoskeleton in most sponges, and is the only skeleton in soft sponges that encrust hard surfaces such as rocks. More commonly the mesohyl is stiffened by mineral spicules, by spongin fibers or both. Spicules, which are present in most but not all species,[28] may be made of silica or calcium carbonate, and vary in shape from simple rods to three-dimensional "stars" with up to six rays. Spicules are produced by sclerocyte cells,[18] and may be separate, connected by joints, or fused.[17]
Some sponges also secrete exoskeletons that lie completely outside their organic components. For example, sclerosponges ("hard sponges") have massive calcium carbonate exoskeletons over which the organic matter forms a thin layer with choanocyte chambers in pits in the mineral. These exoskeletons are secreted by the pinacocytes that form the animals' skins.[18]
Vital functions

Movement
Although adult sponges are fundamentally sessile animals, some marine and freshwater species can move across the sea bed at speeds of 1–4 mm (0.039–0.157 in) per day, as a result of amoeba-like movements of pinacocytes and other cells. A few species can contract their whole bodies, and many can close their oscula and ostia. Juveniles drift or swim freely, while adults are stationary.[18]
Respiration, feeding and excretion

Sponges do not have distinct circulatory, respiratory, digestive, and excretory systems – instead, the water flow system supports all these functions. They filter food particles out of the water flowing through them. Particles larger than 50 micrometers cannot enter the ostia and pinacocytes consume them by phagocytosis (engulfing and intracellular digestion). Particles from 0.5 μm to 50 μm are trapped in the ostia, which taper from the outer to inner ends. These particles are consumed by pinacocytes or by archaeocytes which partially extrude themselves through the walls of the ostia. Bacteria-sized particles, below 0.5 micrometers, pass through the ostia and are caught and consumed by choanocytes.[18] Since the smallest particles are by far the most common, choanocytes typically capture 80% of a sponge's food supply.[29] Archaeocytes transport food packaged in vesicles from cells that directly digest food to those that do not. At least one species of sponge has internal fibers that function as tracks for use by nutrient-carrying archaeocytes,[18] and these tracks also move inert objects.[20]
It used to be claimed that glass sponges could live on nutrients dissolved in sea water and were very averse to silt.[30] However, a study in 2007 found no evidence of this and concluded that they extract bacteria and other micro-organisms from water very efficiently (about 79%) and process suspended sediment grains to extract such prey.[31] Collar bodies digest food and distribute it wrapped in vesicles that are transported by dynein "motor" molecules along bundles of microtubules that run throughout the syncytium.[18]
Sponges' cells absorb oxygen by diffusion from water into cells as water flows through body, into which carbon dioxide and other soluble waste products such as ammonia also diffuse. Archeocytes remove mineral particles that threaten to block the ostia, transport them through the mesohyl and generally dump them into the outgoing water current, although some species incorporate them into their skeletons.[18]
Carnivorous sponges

In waters where the supply of food particles is very poor, some species prey on crustaceans and other small animals. So far only 137 species have been discovered.[33] Most belong to the family Cladorhizidae, but a few members of the Guitarridae and Esperiopsidae are also carnivores.[34] In most cases, little is known about how they actually capture prey, although some species are thought to use either sticky threads or hooked spicules.[34][35] Most carnivorous sponges live in deep waters, up to 8,840 m (5.49 mi),[36] and the development of deep-ocean exploration techniques is expected to lead to the discovery of several more.[18][34] However, one species has been found in Mediterranean caves at depths of 17–23 m (56–75 ft), alongside the more usual filter-feeding sponges. The cave-dwelling predators capture crustaceans under 1 mm (0.039 in) long by entangling them with fine threads, digest them by enveloping them with further threads over the course of a few days, and then return to their normal shape; there is no evidence that they use venom.[36]
Most known carnivorous sponges have completely lost the water flow system and choanocytes. However, the genus Chondrocladia uses a highly modified water flow system to inflate balloon-like structures that are used for capturing prey.[34][37]
Endosymbionts
Freshwater sponges often host green algae as endosymbionts within archaeocytes and other cells and benefit from nutrients produced by the algae. Many marine species host other photosynthesizing organisms, most commonly cyanobacteria but in some cases dinoflagellates. Symbiotic cyanobacteria may form a third of the total mass of living tissue in some sponges, and some sponges gain 48% to 80% of their energy supply from these micro-organisms.[18] In 2008, a University of Stuttgart team reported that spicules made of silica conduct light into the mesohyl, where the photosynthesizing endosymbionts live.[38] Sponges that host photosynthesizing organisms are most common in waters with relatively poor supplies of food particles and often have leafy shapes that maximize the amount of sunlight they collect.[20]
A recently discovered carnivorous sponge that lives near hydrothermal vents hosts methane-eating bacteria and digests some of them.[20]
"Immune" system
Sponges do not have the complex immune systems of most other animals. However, they reject grafts from other species but accept them from other members of their own species. In a few marine species, gray cells play the leading role in rejection of foreign material. When invaded, they produce a chemical that stops movement of other cells in the affected area, thus preventing the intruder from using the sponge's internal transport systems. If the intrusion persists, the grey cells concentrate in the area and release toxins that kill all cells in the area. The "immune" system can stay in this activated state for up to three weeks.[20]
Reproduction
Asexual

Sponges have three asexual methods of reproduction: after fragmentation, by budding, and by producing gemmules. Fragments of sponges may be detached by currents or waves. They use the mobility of their pinacocytes and choanocytes and reshaping of the mesohyl to re-attach themselves to a suitable surface and then rebuild themselves as small but functional sponges over the course of several days. The same capabilities enable sponges that have been squeezed through a fine cloth to regenerate.[18]: 239 A sponge fragment can only regenerate if it contains both collencytes to produce mesohyl and archeocytes to produce all the other cell types.[29] A very few species reproduce by budding.[18]: 90–94
Gemmules are "survival pods" which a few marine sponges and many freshwater species produce by the thousands when dying and which some, mainly freshwater species, regularly produce in autumn. Spongocytes make gemmules by wrapping shells of spongin, often reinforced with spicules, round clusters of archeocytes that are full of nutrients.[18]: 87–88 Freshwater gemmules may also include photosynthesizing symbionts.[39] The gemmules then become dormant, and in this state can survive cold, drying out, lack of oxygen and extreme variations in salinity.[18] Freshwater gemmules often do not revive until the temperature drops, stays cold for a few months and then reaches a near-"normal" level.[39] When a gemmule germinates, the archeocytes round the outside of the cluster transform into pinacocytes, a membrane over a pore in the shell bursts, the cluster of cells slowly emerges, and most of the remaining archeocytes transform into other cell types needed to make a functioning sponge. Gemmules from the same species but different individuals can join forces to form one sponge.[18]: 89–90 Some gemmules are retained within the parent sponge, and in spring it can be difficult to tell whether an old sponge has revived or been "recolonized" by its own gemmules.[39]
Sexual
Most sponges are hermaphrodites (function as both sexes simultaneously), although sponges have no gonads (reproductive organs). Sperm are produced by choanocytes or entire choanocyte chambers that sink into the mesohyl and form spermatic cysts while eggs are formed by transformation of archeocytes, or of choanocytes in some species. Each egg generally acquires a yolk by consuming "nurse cells". During spawning, sperm burst out of their cysts and are expelled via the osculum. If they contact another sponge of the same species, the water flow carries them to choanocytes that engulf them but, instead of digesting them, metamorphose to an ameboid form and carry the sperm through the mesohyl to eggs, which in most cases engulf the carrier and its cargo.[18]: 77
A few species release fertilized eggs into the water, but most retain the eggs until they hatch. By retaining the eggs, the parents can transfer symbiotic microorganisms directly to their offspring through vertical transmission, while the species who release their eggs into the water has to acquire symbionts horizontally (a combination of both is probably most common, where larvae with vertically transmitted symbionts also acquire others horizontally).[40][41] There are four types of larvae, but all are lecithotrophic (non-feeding) balls of cells with an outer layer of cells whose flagella or cilia enable the larvae to move. After swimming for a few days the larvae sink and crawl until they find a place to settle. Most of the cells transform into archeocytes and then into the types appropriate for their locations in a miniature adult sponge.[18]: 77 [42]
Glass sponge embryos start by dividing into separate cells, but once 32 cells have formed they rapidly transform into larvae that externally are ovoid with a band of cilia round the middle that they use for movement, but internally have the typical glass sponge structure of spicules with a cobweb-like main syncitium draped around and between them and choanosyncytia with multiple collar bodies in the center. The larvae then leave their parents' bodies.[43]
Meiosis
The cytological progression of porifera oogenesis and spermatogenesis (gametogenesis) is very similar to that of other metazoa.[44] Most of the genes from the classic set of meiotic genes, including genes for DNA recombination and double-strand break repair, that are conserved in eukaryotes are expressed in the sponges (e.g. Geodia hentscheli and Geodia phlegraei).[44] Since porifera are considered to be the earliest divergent animals, these findings indicate that the basic toolkit of meiosis including capabilities for recombination and DNA repair were present early in eukaryote evolution.[44]
Life cycle
Sponges in temperate regions live for at most a few years, but some tropical species and perhaps some deep-ocean ones may live for 200 years or more. Some calcified demosponges grow by only 0.2 mm (0.0079 in) per year and, if that rate is constant, specimens 1 m (3.3 ft) wide must be about 5,000 years old. Some sponges start sexual reproduction when only a few weeks old, while others wait until they are several years old.[18]
Coordination of activities
Adult sponges lack neurons or any other kind of nervous tissue. However, most species have the ability to perform movements that are coordinated all over their bodies, mainly contractions of the pinacocytes, squeezing the water channels and thus expelling excess sediment and other substances that may cause blockages. Some species can contract the osculum independently of the rest of the body. Sponges may also contract in order to reduce the area that is vulnerable to attack by predators. In cases where two sponges are fused, for example if there is a large but still unseparated bud, these contraction waves slowly become coordinated in both of the "Siamese twins". The coordinating mechanism is unknown, but may involve chemicals similar to neurotransmitters.[45] However, glass sponges rapidly transmit electrical impulses through all parts of the syncytium, and use this to halt the motion of their flagella if the incoming water contains toxins or excessive sediment.[18] Myocytes are thought to be responsible for closing the osculum and for transmitting signals between different parts of the body.[20]
Sponges contain genes very similar to those that contain the "recipe" for the post-synaptic density, an important signal-receiving structure in the neurons of all other animals. However, in sponges these genes are only activated in "flask cells" that appear only in larvae and may provide some sensory capability while the larvae are swimming. This raises questions about whether flask cells represent the predecessors of true neurons or are evidence that sponges' ancestors had true neurons but lost them as they adapted to a sessile lifestyle.[46]
Ecology
Habitats

Sponges are worldwide in their distribution, living in a wide range of ocean habitats, from the polar regions to the tropics.[29] Most live in quiet, clear waters, because sediment stirred up by waves or currents would block their pores, making it difficult for them to feed and breathe.[30] The greatest numbers of sponges are usually found on firm surfaces such as rocks, but some sponges can attach themselves to soft sediment by means of a root-like base.[47]
Sponges are more abundant but less diverse in temperate waters than in tropical waters, possibly because organisms that prey on sponges are more abundant in tropical waters.[48] Glass sponges are the most common in polar waters and in the depths of temperate and tropical seas, as their very porous construction enables them to extract food from these resource-poor waters with the minimum of effort. Demosponges and calcareous sponges are abundant and diverse in shallower non-polar waters.[49]
The different classes of sponge live in different ranges of habitat:
Class Water type[20] Depth[20] Type of surface[20] Calcarea Marine less than 100 m (330 ft) Hard Glass sponges Marine Deep Soft or firm sediment Demosponges Marine, brackish; and about 150 freshwater species[18] Inter-tidal to abyssal;[20] a carnivorous demosponge has been found at 8,840 m (5.49 mi)[36] Any
As primary producers
Sponges with photosynthesizing endosymbionts produce up to three times more oxygen than they consume, as well as more organic matter than they consume. Such contributions to their habitats' resources are significant along Australia's Great Barrier Reef but relatively minor in the Caribbean.[29]
Defenses


Many sponges shed spicules, forming a dense carpet several meters deep that keeps away echinoderms which would otherwise prey on the sponges.[29] They also produce toxins that prevent other sessile organisms such as bryozoans or sea squirts from growing on or near them, making sponges very effective competitors for living space. One of many examples includes ageliferin.
A few species, the Caribbean fire sponge Tedania ignis, cause a severe rash in humans who handle them.[18] Turtles and some fish feed mainly on sponges. It is often said that sponges produce chemical defenses against such predators.[18] However, experiments have been unable to establish a relationship between the toxicity of chemicals produced by sponges and how they taste to fish, which would diminish the usefulness of chemical defenses as deterrents. Predation by fish may even help to spread sponges by detaching fragments.[20] However, some studies have shown fish showing a preference for non chemically defended sponges,[50] and another study found that high levels of coral predation did predict the presence of chemically defended species.[51]
Glass sponges produce no toxic chemicals, and live in very deep water where predators are rare.[30]
Predation
Spongeflies, also known as spongillaflies (Neuroptera, Sisyridae), are specialist predators of freshwater sponges. The female lays her eggs on vegetation overhanging water. The larvae hatch and drop into the water where they seek out sponges to feed on. They use their elongated mouthparts to pierce the sponge and suck the fluids within. The larvae of some species cling to the surface of the sponge while others take refuge in the sponge's internal cavities. The fully grown larvae leave the water and spin a cocoon in which to pupate.[52]
Bioerosion
The Caribbean chicken-liver sponge Chondrilla nucula secretes toxins that kill coral polyps, allowing the sponges to grow over the coral skeletons.[18] Others, especially in the family Clionaidae, use corrosive substances secreted by their archeocytes to tunnel into rocks, corals and the shells of dead mollusks.[18] Sponges may remove up to 1 m (3.3 ft) per year from reefs, creating visible notches just below low-tide level.[29]
Diseases
Caribbean sponges of the genus Aplysina suffer from Aplysina red band syndrome. This causes Aplysina to develop one or more rust-colored bands, sometimes with adjacent bands of necrotic tissue. These lesions may completely encircle branches of the sponge. The disease appears to be contagious and impacts approximately 10 percent of A. cauliformis on Bahamian reefs.[53] The rust-colored bands are caused by a cyanobacterium, but it is unknown whether this organism actually causes the disease.[53][54]
Collaboration with other organisms
In addition to hosting photosynthesizing endosymbionts,[18] sponges are noted for their wide range of collaborations with other organisms. The relatively large encrusting sponge Lissodendoryx colombiensis is most common on rocky surfaces, but has extended its range into seagrass meadows by letting itself be surrounded or overgrown by seagrass sponges, which are distasteful to the local starfish and therefore protect Lissodendoryx against them; in return, the seagrass sponges get higher positions away from the sea-floor sediment.[55]
Shrimps of the genus Synalpheus form colonies in sponges, and each shrimp species inhabits a different sponge species, making Synalpheus one of the most diverse crustacean genera. Specifically, Synalpheus regalis utilizes the sponge not only as a food source, but also as a defense against other shrimp and predators.[56] As many as 16,000 individuals inhabit a single loggerhead sponge, feeding off the larger particles that collect on the sponge as it filters the ocean to feed itself.[57] Other crustaceans such as hermit crabs commonly have a specific species of sponge, Pseudospongosorites, grow on them as both the sponge and crab occupy gastropod shells until the crab and sponge outgrow the shell, eventually resulting in the crab using the sponge's body as protection instead of the shell until the crab finds a suitable replacement shell.[58]


Sponge loop
Most sponges are detritivores which filter organic debris particles and microscopic life forms from ocean water. In particular, sponges occupy an important role as detritivores in coral reef food webs by recycling detritus to higher trophic levels.[61]
The hypothesis has been made that coral reef sponges facilitate the transfer of coral-derived organic matter to their associated detritivores via the production of sponge detritus, as shown in the diagram. Several sponge species are able to convert coral-derived DOM into sponge detritus,[62][63] and transfer organic matter produced by corals further up the reef food web. Corals release organic matter as both dissolved and particulate mucus,[64][65][66][67] as well as cellular material such as expelled Symbiodinium.[68][69][61]
Organic matter could be transferred from corals to sponges by all these pathways, but DOM likely makes up the largest fraction, as the majority (56 to 80%) of coral mucus dissolves in the water column,[65] and coral loss of fixed carbon due to expulsion of Symbiodinium is typically negligible (0.01%)[68] compared with mucus release (up to ~40%).[70][71] Coral-derived organic matter could also be indirectly transferred to sponges via bacteria, which can also consume coral mucus.[72][73][74][61]


Sponge holobiont
Besides a one to one symbiotic relationship, it is possible for a host to become symbiotic with a microbial consortium, resulting in a diverse sponge microbiome. Sponges are able to host a wide range of microbial communities that can also be very specific. The microbial communities that form a symbiotic relationship with the sponge can amount to as much as 35% of the biomass of its host.[77] The term for this specific symbiotic relationship, where a microbial consortia pairs with a host is called a holobiotic relationship. The sponge as well as the microbial community associated with it will produce a large range of secondary metabolites that help protect it against predators through mechanisms such as chemical defense.[78]
Some of these relationships include endosymbionts within bacteriocyte cells, and cyanobacteria or microalgae found below the pinacoderm cell layer where they are able to receive the highest amount of light, used for phototrophy. They can host over 50 different microbial phyla and candidate phyla, including Alphaprotoebacteria, Actinomycetota, Chloroflexota, Nitrospirota, "Cyanobacteria", the taxa Gamma-, the candidate phylum Poribacteria, and Thaumarchaea.[78]
Systematics
Taxonomy
Carl Linnaeus, who classified most kinds of sessile animals as belonging to the order Zoophyta in the class Vermes, mistakenly identified the genus Spongia as plants in the order Algae.[79][further explanation needed] For a long time thereafter, sponges were assigned to subkingdom Parazoa ("beside the animals") separated from the Eumetazoa which formed the rest of the kingdom Animalia.[80]
The phylum Porifera is further divided into classes mainly according to the composition of their skeletons:[17][29]
- Hexactinellida (glass sponges) have silicate spicules, the largest of which have six rays and may be individual or fused.[17] The main components of their bodies are syncytia in which large numbers of cell share a single external membrane.[29]
- Calcarea have skeletons made of calcite, a form of calcium carbonate, which may form separate spicules or large masses. All the cells have a single nucleus and membrane.[29]
- Most Demospongiae have silicate spicules or spongin fibers or both within their soft tissues. However, a few also have massive external skeletons made of aragonite, another form of calcium carbonate.[17][29] All the cells have a single nucleus and membrane.[29]
- Archeocyatha are known only as fossils from the Cambrian period.[80]
In the 1970s, sponges with massive calcium carbonate skeletons were assigned to a separate class, Sclerospongiae, otherwise known as "coralline sponges".[81] However, in the 1980s it was found that these were all members of either the Calcarea or the Demospongiae.[82]
So far scientific publications have identified about 9,000 poriferan species,[29] of which: about 400 are glass sponges; about 500 are calcareous species; and the rest are demosponges.[18] However, some types of habitat, vertical rock and cave walls and galleries in rock and coral boulders, have been investigated very little, even in shallow seas.[29]
Classes
Sponges were traditionally distributed in three classes: calcareous sponges (Calcarea), glass sponges (Hexactinellida) and demosponges (Demospongiae). However, studies have now shown that the Homoscleromorpha, a group thought to belong to the Demospongiae, has a genetic relationship well separated from other sponge classes.[13]: 153–154 Therefore, they have recently been recognized as the fourth class of sponges.[83][84]
Sponges are divided into classes mainly according to the composition of their skeletons:[20] These are arranged in evolutionary order as shown below in ascending order of their evolution from top to bottom:
Class Type of cells[20] Spicules[20] Spongin fibers[20] Massive exoskeleton[29] Body form[20] Hexactinellida Mostly syncytia in all species Silica
May be individual or fusedNever Never Leuconoid Demospongiae Single nucleus, single external membrane Silica In many species In some species.
Made of aragonite if present.[17][29]Leuconoid Calcarea Single nucleus, single external membrane Calcite
May be individual or large massesNever Common.
Made of calcite if present.Asconoid, syconoid, leuconoid or solenoid[85] Homoscleromorpha Single nucleus, single external membrane Silica In many species Never Sylleibid or leuconoid
Phylogeny
The phylogeny of sponges has been debated heavily since the advent of phylogenetics. Originally thought to be the most basal animal phylum, there is now considerable evidence that Ctenophora may hold that title instead.[86][87] Additionally, the monophyly of the phylum is now under question. Several studies have concluded that all other animals emerged from within the sponges, and usually recover that the calcareous sponges and Homoscleromorpha are closer to other animals than to demosponges.[88][89] The internal relationships of Porifera have proven to be less uncertain. A close relationship of Homoscleromorpha and Calcarea has been recovered in nearly all studies, whether or not they support sponge or eumetazoan monophyly.[88][6][84][83] The position of glass sponges is also fairly certain, with a majority of studies recovering them as the sister of the demosponges.[83][6][88] Thus, the uncertainty at the base of the animal family tree is probably best represented by the below cladogram.
Evolutionary history
Fossil record


Although molecular clocks and biomarkers suggest sponges existed well before the Cambrian explosion of life, silica spicules like those of demosponges are absent from the fossil record until the Cambrian.[90] An unsubstantiated 2002 report exists of spicules in rocks dated around 750 million years ago.[91] Well-preserved fossil sponges from about 580 million years ago in the Ediacaran period have been found in the Doushantuo Formation.[92] These fossils, which include: spicules; pinacocytes; porocytes; archeocytes; sclerocytes; and the internal cavity, have been classified as demosponges. Fossils of glass sponges have been found from around 540 million years ago in rocks in Australia, China, and Mongolia.[93] Early Cambrian sponges from Mexico belonging to the genus Kiwetinokia show evidence of fusion of several smaller spicules to form a single large spicule.[94] Calcium carbonate spicules of calcareous sponges have been found in Early Cambrian rocks from about 530 to 523 million years ago in Australia. Other probable demosponges have been found in the Early Cambrian Chengjiang fauna, from 525 to 520 million years ago.[95] Fossils found in the Canadian Northwest Territories dating to 890 million years ago may be sponges; if this finding is confirmed, it suggests the first animals appeared before the Neoproterozoic oxygenation event.[96]

Freshwater sponges appear to be much younger, as the earliest known fossils date from the Mid-Eocene period about 48 to 40 million years ago.[93] Although about 90% of modern sponges are demosponges, fossilized remains of this type are less common than those of other types because their skeletons are composed of relatively soft spongin that does not fossilize well.[97] The earliest sponge symbionts are known from the early Silurian.[98]
A chemical tracer is 24-isopropylcholestane, which is a stable derivative of 24-isopropylcholesterol, which is said to be produced by demosponges but not by eumetazoans ("true animals", i.e. cnidarians and bilaterians). Since choanoflagellates are thought to be animals' closest single-celled relatives, a team of scientists examined the biochemistry and genes of one choanoflagellate species. They concluded that this species could not produce 24-isopropylcholesterol but that investigation of a wider range of choanoflagellates would be necessary in order to prove that the fossil 24-isopropylcholestane could only have been produced by demosponges.[99] Although a previous publication reported traces of the chemical 24-isopropylcholestane in ancient rocks dating to 1,800 million years ago,[100] recent research using a much more accurately dated rock series has revealed that these biomarkers only appear before the end of the Marinoan glaciation approximately 635 million years ago,[101] and that "Biomarker analysis has yet to reveal any convincing evidence for ancient sponges pre-dating the first globally extensive Neoproterozoic glacial episode (the Sturtian, ~713 million years ago in Oman)". While it has been argued that this 'sponge biomarker' could have originated from marine algae, recent research suggests that the algae's ability to produce this biomarker evolved only in the Carboniferous; as such, the biomarker remains strongly supportive of the presence of demosponges in the Cryogenian.[102][103][104]
Archaeocyathids, which some classify as a type of coralline sponge, are very common fossils in rocks from the Early Cambrian about 530 to 520 million years ago, but apparently died out by the end of the Cambrian 490 million years ago.[95] It has been suggested that they were produced by: sponges; cnidarians; algae; foraminiferans; a completely separate phylum of animals, Archaeocyatha; or even a completely separate kingdom of life, labeled Archaeata or Inferibionta. Since the 1990s archaeocyathids have been regarded as a distinctive group of sponges.[80]
It is difficult to fit chancelloriids into classifications of sponges or more complex animals. An analysis in 1996 concluded that they were closely related to sponges on the grounds that the detailed structure of chancellorid sclerites ("armor plates") is similar to that of fibers of spongin, a collagen protein, in modern keratose (horny) demosponges such as Darwinella.[106] However, another analysis in 2002 concluded that chancelloriids are not sponges and may be intermediate between sponges and more complex animals, among other reasons because their skins were thicker and more tightly connected than those of sponges.[107] In 2008, a detailed analysis of chancelloriids' sclerites concluded that they were very similar to those of halkieriids, mobile bilaterian animals that looked like slugs in chain mail and whose fossils are found in rocks from the very Early Cambrian to the Mid Cambrian. If this is correct, it would create a dilemma, as it is extremely unlikely that totally unrelated organisms could have developed such similar sclerites independently, but the huge difference in the structures of their bodies makes it hard to see how they could be closely related.[105]
Relationships to other animal groups

| Simplified family tree showing calcareous sponges as closest to more complex animals[108] |
| Simplified family tree showing Homoscleromorpha as closest to more complex animals[109] |
In the 1990s, sponges were widely regarded as a monophyletic group, all of them having descended from a common ancestor that was itself a sponge, and as the "sister-group" to all other metazoans (multi-celled animals), which themselves form a monophyletic group. On the other hand, some 1990s analyses also revived the idea that animals' nearest evolutionary relatives are choanoflagellates, single-celled organisms very similar to sponges' choanocytes – which would imply that most Metazoa evolved from very sponge-like ancestors and therefore that sponges may not be monophyletic, as the same sponge-like ancestors may have given rise both to modern sponges and to non-sponge members of Metazoa.[108]
Analyses since 2001 have concluded that Eumetazoa (more complex than sponges) are more closely related to particular groups of sponges than to other sponge groups. Such conclusions imply that sponges are not monophyletic, because the last common ancestor of all sponges would also be a direct ancestor of the Eumetazoa, which are not sponges. A study in 2001 based on comparisons of ribosome DNA concluded that the most fundamental division within sponges was between glass sponges and the rest, and that Eumetazoa are more closely related to calcareous sponges (those with calcium carbonate spicules) than to other types of sponge.[108] In 2007, one analysis based on comparisons of RNA and another based mainly on comparison of spicules concluded that demosponges and glass sponges are more closely related to each other than either is to the calcareous sponges, which in turn are more closely related to Eumetazoa.[93][110]
Other anatomical and biochemical evidence links the Eumetazoa with Homoscleromorpha, a sub-group of demosponges. A comparison in 2007 of nuclear DNA, excluding glass sponges and comb jellies, concluded that:
- Homoscleromorpha are most closely related to Eumetazoa;
- calcareous sponges are the next closest;
- the other demosponges are evolutionary "aunts" of these groups; and
- the chancelloriids, bag-like animals whose fossils are found in Cambrian rocks, may be sponges.[109]
The sperm of Homoscleromorpha share features with the sperm of Eumetazoa, that sperm of other sponges lack. In both Homoscleromorpha and Eumetazoa layers of cells are bound together by attachment to a carpet-like basal membrane composed mainly of "typ IV" collagen, a form of collagen not found in other sponges – although the spongin fibers that reinforce the mesohyl of all demosponges is similar to "type IV" collagen.[22]

The analyses described above concluded that sponges are closest to the ancestors of all Metazoa, of all multi-celled animals including both sponges and more complex groups. However, another comparison in 2008 of 150 genes in each of 21 genera, ranging from fungi to humans but including only two species of sponge, suggested that comb jellies (ctenophora) are the most basal lineage of the Metazoa included in the sample.[111][112][113][114] If this is correct, either modern comb jellies developed their complex structures independently of other Metazoa, or sponges' ancestors were more complex and all known sponges are drastically simplified forms. The study recommended further analyses using a wider range of sponges and other simple Metazoa such as Placozoa.[111]
However, reanalysis of the data showed that the computer algorithms used for analysis were misled by the presence of specific ctenophore genes that were markedly different from those of other species, leaving sponges as either the sister group to all other animals, or an ancestral paraphyletic grade.[115][116] 'Family trees' constructed using a combination of all available data – morphological, developmental and molecular – concluded that the sponges are in fact a monophyletic group, and with the cnidarians form the sister group to the bilaterians.[117][118]
A very large and internally consistent alignment of 1,719 proteins at the metazoan scale, published in 2017, showed that (i) sponges – represented by Homoscleromorpha, Calcarea, Hexactinellida, and Demospongiae – are monophyletic, (ii) sponges are sister-group to all other multicellular animals, (iii) ctenophores emerge as the second-earliest branching animal lineage, and (iv) placozoans emerge as the third animal lineage, followed by cnidarians sister-group to bilaterians.[119]
In March 2021, scientists from Dublin found additional evidence that sponges are the sister group to all other animals,[120] while in May 2023, Schultz et al. found patterns of irreversible change in genome synteny that provide strong evidence that ctenophores are the sister group to all other animals instead.[121]
Notable spongiologists
- Céline Allewaert
- Patricia Bergquist
- James Scott Bowerbank
- Maurice Burton
- Henry John Carter
- Max Walker de Laubenfels
- Arthur Dendy
- Édouard Placide Duchassaing de Fontbressin
- Randolph Kirkpatrick
- Robert J. Lendlmayer von Lendenfeld
- Edward Alfred Minchin
- Giovanni Domenico Nardo
- Eduard Oscar Schmidt
- Émile Topsent
Use

By dolphins
A report in 1997 described use of sponges as a tool by bottlenose dolphins in Shark Bay in Western Australia. A dolphin will attach a marine sponge to its rostrum, which is presumably then used to protect it when searching for food in the sandy sea bottom.[122] The behavior, known as sponging, has only been observed in this bay and is almost exclusively shown by females. A study in 2005 concluded that mothers teach the behavior to their daughters and that all the sponge users are closely related, suggesting that it is a fairly recent innovation.[16]
By humans


Skeleton
The calcium carbonate or silica spicules of most sponge genera make them too rough for most uses, but two genera, Hippospongia and Spongia, have soft, entirely fibrous skeletons.[13]: 88 Early Europeans used soft sponges for many purposes, including padding for helmets, portable drinking utensils and municipal water filters. Until the invention of synthetic sponges, they were used as cleaning tools, applicators for paints and ceramic glazes and discreet contraceptives. However, by the mid-20th century, overfishing brought both the animals and the industry close to extinction.[123]
Many objects with sponge-like textures are now made of substances not derived from poriferans. Synthetic sponges include personal and household cleaning tools, breast implants,[124] and contraceptive sponges.[125] Typical materials used are cellulose foam, polyurethane foam, and less frequently, silicone foam.
The luffa "sponge", also spelled loofah, which is commonly sold for use in the kitchen or the shower, is not derived from an animal but mainly from the fibrous "skeleton" of the sponge gourd (Luffa aegyptiaca, Cucurbitaceae).[126]
Antibiotic compounds
Sponges have medicinal potential due to the presence in sponges themselves or their microbial symbionts of chemicals that may be used to control viruses, bacteria, tumors and fungi.[127][128]
Other biologically active compounds

Lacking any protective shell or means of escape, sponges have evolved to synthesize a variety of unusual compounds. One such class is the oxidized fatty acid derivatives called oxylipins. Members of this family have been found to have anti-cancer, anti-bacterial and anti-fungal properties. One example isolated from the Okinawan plakortis sponges, plakoridine A, has shown potential as a cytotoxin to murine lymphoma cells.[129][130]
See also
- Lists of sponges
- Sponge Reef Project
- 3-Alkylpyridinium, compounds found in marine Haplosclerida sponges
References
- ^ Brasier, Martin; Green, Owen; Shields, Graham (April 1997). "Ediacarian sponge spicule clusters from southwestern Mongolia and the origins of the Cambrian fauna" (PDF). Geology. 25 (4): 303–306. Bibcode:1997Geo....25..303B. doi:10.1130/0091-7613(1997)025<0303:ESSCFS>2.3.CO;2.
- ^ Gold, David; Grabenstatter, Jonathan; de Mendoza, Alex; Riesgo, Ana; Ruiz-Trillo, Iñaki; Summons, Roger (22 February 2016). "Sterol and genomic analyses validate the sponge biomarker hypothesis". PNAS. doi:10.1073/pnas.1512614113.
- ^ Pajdzińska, A. (2018). "Animals die more shallowly: they aren't deceased, they're dead. Animals in the polish linguistic worldview and in contemporary life sciences". Ethnolinguistic. 29: 147–161. doi:10.17951/et.2017.29.135.
- ^ "Porifera". World Register of Marine Species. Flanders Marine Institute. 2024. Retrieved 8 May 2024.
- ^ "porifera". Merriam-Webster.com Dictionary. Merriam-Webster.
- ^ a b c d Feuda, Roberto; Dohrmann, Martin; Pett, Walker; Philippe, Hervé; Rota-Stabelli, Omar; Lartillot, Nicolas; Wörheide, Gert; Pisani, Davide (December 2017). "Improved Modeling of Compositional Heterogeneity Supports Sponges as Sister to All Other Animals". Current Biology. 27 (24): 3864–3870.e4. Bibcode:2017CBio...27E3864F. doi:10.1016/j.cub.2017.11.008. PMID 29199080.
- ^ a b Moroz, L.L.; Romanova, D.Y. (23 December 2022). "Alternative neural systems: What is a neuron? (Ctenophores, sponges and placozoans)". Frontiers in Cell and Developmental Biology. 10: 1071961. doi:10.3389/fcell.2022.1071961. PMC 9816575. PMID 36619868.
- ^ "Spongiology". Merriam-Webster.com Dictionary. Merriam-Webster. Retrieved 27 December 2017.
- ^ Liddell, Henry George; Scott, Robert (1940). "σπόγγος". A Greek-English Lexicon. Archived from the original on 5 September 2021. Retrieved 5 September 2021 – via Perseus.
- ^ a b Hooper, J. (2018). "Structure of Sponges". Queensland Museum. Archived from the original on 26 September 2019. Retrieved 27 September 2019.
- ^ Thacker, Robert W.; Díaz, Maria Cristina; Kerner, Adeline; Vignes-Lebbe, Régine; Segerdell, Erik; Haendel, Melissa A.; Mungall, Christopher J. (8 September 2014). "The Porifera Ontology (PORO): enhancing sponge systematics with an anatomy ontology". Journal of Biomedical Semantics. 5 (1): 39. doi:10.1186/2041-1480-5-39. PMC 4177528. PMID 25276334.
- ^ Collins, A.G. (December 1998). "Evaluating multiple alternative hypotheses for the origin of Bilateria: an analysis of 18S rRNA molecular evidence". Proceedings of the National Academy of Sciences of the United States of America. 95 (26): 15458–63. Bibcode:1998PNAS...9515458C. doi:10.1073/pnas.95.26.15458. PMC 28064. PMID 9860990.
- ^ a b c d e f g Bergquist, P. R. (1978). Sponges. London: Hutchinson. ISBN 978-0-520-03658-1.
- ^ Vacelet, J.; Duport, E. (2004). "Prey capture and digestion in the carnivorous sponge Asbestopluma hypogea (Porifera: Demospongiae)". Zoomorphology. 123 (4): 179–190. doi:10.1007/s00435-004-0100-0. S2CID 24484610.
- ^ Clark, M.A.; Choi, J.; Douglas, M. (2018) Biology 2e[permanent dead link], page 776, OpenStax. ISBN 978-1-947172-52-4.
- ^ a b Krützen, Michael; Mann, Janet; Heithaus, Michael R.; Connor, Richard C.; Bejder, Lars; Sherwin, William B. (June 2005). "Cultural transmission of tool use in bottlenose dolphins". Proceedings of the National Academy of Sciences of the United States of America. 102 (25): 8939–43. Bibcode:2005PNAS..102.8939K. doi:10.1073/pnas.0500232102. PMC 1157020. PMID 15947077.
- ^ a b c d e f g Hooper, J. N. A.; Van Soest, R. W. M.; Debrenne, F. (2002). "Phylum Porifera Grant, 1836". In Hooper, J. N. A.; Van Soest, R. W. M. (eds.). Systema Porifera: A Guide to the Classification of Sponges. New York: Kluwer Academic/Plenum. pp. 9–14. ISBN 978-0-306-47260-2.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak Ruppert, Edward E.; Fox, Richard S.; Barnes, Robert D. (2004). Invertebrate Zoology (7th ed.). Brooks / COLE Publishing. pp. 76–97. ISBN 978-0-03-025982-1.
- ^ Bakshani, Cassie R.; Morales-Garcia, Ana L.; Althaus, Mike; Wilcox, Matthew D.; Pearson, Jeffrey P.; Bythell, John C.; Burgess, J. Grant (2018-07-04). "Evolutionary conservation of the antimicrobial function of mucus: a first defence against infection". npj Biofilms and Microbiomes. 4 (1): 14. doi:10.1038/s41522-018-0057-2. PMC 6031612. PMID 30002868.
- ^ a b c d e f g h i j k l m n o p q r s t Bergquist, P.R. (1998). "Porifera". In Anderson, D.T. (ed.). Invertebrate Zoology. Oxford University Press. pp. 10–27. ISBN 978-0-19-551368-4.
- ^ a b c Hinde, R. T. (1998). "The Cnidaria and Ctenophora". In Anderson, D. T. (ed.). Invertebrate Zoology. Oxford University Press. pp. 28–57. ISBN 978-0-19-551368-4.
- ^ a b Exposito, Jean-Yves; Cluzel, Caroline; Garrone, Robert; Lethias, Claire (November 2002). "Evolution of collagens". The Anatomical Record. 268 (3): 302–16. doi:10.1002/ar.10162. PMID 12382326.
- ^ Ruppert EE, Fox RS, Barnes RD (2004). Invertebrate Zoology (7th ed.). Brooks / Cole. p. 82. ISBN 978-0-03-025982-1.
- ^ Musser, Jacob M.; Schippers, Klaske J.; Nickel, Michael; Mizzon, Giulia; Kohn, Andrea B.; Pape, Constantin; et al. (November 2021). "Profiling cellular diversity in sponges informs animal cell type and nervous system evolution". Science. 374 (6568): 717–723. Bibcode:2021Sci...374..717M. doi:10.1126/science.abj2949. PMC 9233960. PMID 34735222.
- ^ Rivera, A.S.; Ozturk, N.; Fahey, B.; Plachetzki, D.C.; Degnan, B.M.; Sancar, A.; Oakley, T.H. (April 2012). "Blue-light-receptive cryptochrome is expressed in a sponge eye lacking neurons and opsin". The Journal of Experimental Biology. 215 (Pt 8): 1278–86. doi:10.1242/jeb.067140. PMC 3309880. PMID 22442365.
- ^ Ruppert, Edward E.; Fox, Richard S.; Barnes, Robert D. (2004). Invertebrate Zoology (7th ed.). Brooks / Cole. p. 78. ISBN 978-0-03-025982-1.
- ^ Hickman, C.P.; Roberts, L.S.; Larson, A. (2001). Integrated Principles of Zoology (11th ed.). New York: McGraw-Hill. p. 247. ISBN 978-0-07-290961-6.
- ^ "Marine Species Identification Portal: Halisarca dujardini". species-identification.org. Archived from the original on 2020-10-17. Retrieved 2019-08-02.
- ^ a b c d e f g h i j k l m n o Bergquist, P.R. (2001). "Porifera (Sponges)". Encyclopedia of Life Sciences. John Wiley & Sons. doi:10.1038/npg.els.0001582. ISBN 978-0-470-01617-6.
- ^ a b c Krautter, M. (1998). "Ecology of siliceous sponges: Application to the environmental interpretation of the Upper Jurassic sponge facies (Oxfordian) from Spain" (PDF). Cuadernos de Geología Ibérica. 24: 223–239. Archived from the original (PDF) on March 19, 2009. Retrieved 2008-10-10.
- ^ Yahel, G.; Whitney, F.; Reiswig, H.M.; Eerkes-Medrano, D.I.; Leys, S.P. (2007). "In situ feeding and metabolism of glass sponges (Hexactinellida, Porifera) studied in a deep temperate fjord with a remotely operated submersible". Limnology and Oceanography. 52 (1): 428–440. Bibcode:2007LimOc..52..428Y. CiteSeerX 10.1.1.597.9627. doi:10.4319/lo.2007.52.1.0428. S2CID 86297053.
- ^ Van Soest, Rob W. M.; Boury-Esnault, Nicole; Vacelet, Jean; Dohrmann, Martin; Erpenbeck, Dirk; De Voogd, Nicole J.; Santodomingo, Nadiezhda; Vanhoorne, Bart; Kelly, Michelle; Hooper, John N. A. (2012). "Global diversity of sponges (Porifera)". PLOS ONE. 7 (4): e35105. Bibcode:2012PLoSO...735105V. doi:10.1371/journal.pone.0035105. PMC 3338747. PMID 22558119.
- ^ "4 new species of 'killer' sponges discovered off Pacific coast". CBC News. April 19, 2014. Archived from the original on April 19, 2014. Retrieved 2014-09-04.
- ^ a b c d Vacelet, J. (2008). "A new genus of carnivorous sponges (Porifera: Poecilosclerida, Cladorhizidae) from the deep N-E Pacific, and remarks on the genus Neocladia" (PDF). Zootaxa. 1752: 57–65. doi:10.11646/zootaxa.1752.1.3. Archived (PDF) from the original on 2008-09-06. Retrieved 2008-10-31.
- ^ Watling, L. (2007). "Predation on copepods by an Alaskan cladorhizid sponge". Journal of the Marine Biological Association of the United Kingdom. 87 (6): 1721–1726. Bibcode:2007JMBUK..87.1721W. doi:10.1017/S0025315407058560. S2CID 86588792.
- ^ a b c Vacelet, J.; Boury-Esnault, N. (1995). "Carnivorous sponges". Nature. 373 (6512): 333–335. Bibcode:1995Natur.373..333V. doi:10.1038/373333a0. S2CID 4320216.
- ^ Vacelet, J.; Kelly, Michelle (2008). "New species from the deep Pacific suggest that carnivorous sponges date back to the Early Jurassic". Nature Precedings. doi:10.1038/npre.2008.2327.1.
- ^ Brümmer, Franz; Pfannkuchen, Martin; Baltz, Alexander; Hauser, Thomas; Thiel, Vera (2008). "Light inside sponges". Journal of Experimental Marine Biology and Ecology. 367 (2): 61–64. Bibcode:2008JEMBE.367...61B. doi:10.1016/j.jembe.2008.06.036.
- Walker, Matt (10 November 2008). "Nature's 'fibre optics' experts". BBC News. Archived from the original on 17 December 2008. Retrieved 11 November 2008.
- ^ a b c Smith, D. G.; Pennak, R. W. (2001). Pennak's Freshwater Invertebrates of the United States: Porifera to Crustacea (4 ed.). John Wiley and Sons. pp. 47–50. ISBN 978-0-471-35837-4.
- ^ Díez-Vives, Cristina; Koutsouveli, Vasiliki; Conejero, Maria; Riesgo, Ana (26 October 2022). "Global patterns in symbiont selection and transmission strategies in sponges". Frontiers in Ecology and Evolution. 10. doi:10.3389/fevo.2022.1015592. ISSN 2296-701X.
- ^ Carrier, Tyler J.; Maldonado, Manuel; Schmittmann, Lara; Pita, Lucía; Bosch, Thomas C. G.; Hentschel, Ute (May 2022). "Symbiont transmission in marine sponges: reproduction, development, and metamorphosis". BMC Biology. 20 (1): 100. doi:10.1186/s12915-022-01291-6. PMC 9077847. PMID 35524305.
- ^ Riesgo, Ana; Taboada, Sergio; Sánchez-Vila, Laura; Solà, Joan; Bertran, Andrea; Avila, Conxita (18 March 2015). "Some Like It Fat: Comparative Ultrastructure of the Embryo in Two Demosponges of the Genus Mycale (Order Poecilosclerida) from Antarctica and the Caribbean". PLOS ONE. 10 (3): e0118805. Bibcode:2015PLoSO..1018805R. doi:10.1371/journal.pone.0118805. ISSN 1932-6203. PMC 4365022. PMID 25785444.
- ^ Leys, S. P. (16 February 2006). "Embryogenesis in the glass sponge Oopsacas minuta: Formation of syncytia by fusion of blastomeres". Integrative and Comparative Biology. 46 (2): 104–117. doi:10.1093/icb/icj016. ISSN 1540-7063. PMID 21672727.
- ^ a b c Koutsouveli, Vasiliki; Cárdenas, Paco; Santodomingo, Nadiezhda; Marina, Anabel; Morato, Esperanza; Rapp, Hans Tore; Riesgo, Ana (16 December 2020). "The Molecular Machinery of Gametogenesis in Geodia Demosponges (Porifera): Evolutionary Origins of a Conserved Toolkit across Animals". Molecular Biology and Evolution. 37 (12): 3485–3506. doi:10.1093/molbev/msaa183. ISSN 0737-4038. PMC 7743902. PMID 32929503.
- ^ Nickel, M. (December 2004). "Kinetics and rhythm of body contractions in the sponge Tethya wilhelma (Porifera: Demospongiae)". The Journal of Experimental Biology. 207 (Pt 26): 4515–24. doi:10.1242/jeb.01289. PMID 15579547.
- ^ Sakarya, Onur; Armstrong, Kathryn A.; Adamska, Maja; Adamski, Marcin; Wang, I-Fan; Tidor, Bruce; Degnan, Bernard M.; Oakley, Todd H.; Kosik, Kenneth S. (6 June 2007). "A Post-Synaptic Scaffold at the Origin of the Animal Kingdom". PLOS ONE. 2 (6): e506. Bibcode:2007PLoSO...2..506S. doi:10.1371/journal.pone.0000506. ISSN 1932-6203. PMC 1876816. PMID 17551586.
- ^ Weaver, James C.; Aizenberg, Joanna; Fantner, Georg E.; Kisailus, David; Woesz, Alexander; Allen, Peter; Fields, Kirk; Porter, Michael J.; Zok, Frank W.; Hansma, Paul K.; Fratzl, Peter; Morse, Daniel E. (2007). "Hierarchical assembly of the siliceous skeletal lattice of the hexactinellid sponge Euplectella aspergillum". Journal of Structural Biology. 158 (1): 93–106. doi:10.1016/j.jsb.2006.10.027. PMID 17175169.
- ^ Ruzicka, R; Gleason, D.F. (January 2008). "Latitudinal variation in spongivorous fishes and the effectiveness of sponge chemical defenses" (PDF). Oecologia. 154 (4): 785–94. Bibcode:2008Oecol.154..785R. doi:10.1007/s00442-007-0874-0. PMID 17960425. S2CID 1495896. Archived from the original (PDF) on 2008-10-06.
- ^ Gage, J.D.; Tyler, P.A. (1996). Deep-sea Biology: A Natural History of Organisms at the Deep-Sea Floor. Cambridge University Press. pp. 91–93. ISBN 978-0-521-33665-9.
- ^ Dunlap, M.; Pawlik, J. R. (1996). "Video-monitored predation by Caribbean reef fishes on an array of mangrove and reef sponges". Marine Biology. 126 (1): 117–123. Bibcode:1996MarBi.126..117D. doi:10.1007/bf00571383. ISSN 0025-3162. S2CID 84799900.
- ^ Loh, T. L.; Pawlik, J. R. (March 2014). "Chemical defenses and resource trade-offs structure sponge communities on Caribbean coral reefs". Proceedings of the National Academy of Sciences of the United States of America. 111 (11): 4151–6. Bibcode:2014PNAS..111.4151L. doi:10.1073/pnas.1321626111. PMC 3964098. PMID 24567392.
- ^ Piper, Ross (2007). Extraordinary Animals: An Encyclopedia of Curious and Unusual Animals. Greenwood Publishing Group. p. 148. ISBN 978-0-313-33922-6.
- ^ a b Gochfeld, D.J.; et al. (2012). Steller, D.; Lobel, L. (eds.). "Population Dynamics of a Sponge Disease on Caribbean Reefs". Diving for Science 2012. Proceedings of the American Academy of Underwater Sciences 31st Symposium. Archived from the original on 2015-09-04.
{{cite journal}}: CS1 maint: unfit URL (link) - ^ Olson, J.B.; Gochfeld, D.J.; Slattery, M. (July 2006). "Aplysina red band syndrome: a new threat to Caribbean sponges". Diseases of Aquatic Organisms. 71 (2): 163–8. doi:10.3354/dao071163. PMID 16956064. Lay summary in: Clarke, M. (2006-10-17). "New disease threatens sponges". Practical Fishkeeping. Archived from the original on 2007-09-26.
- ^ Wulff, J. L. (June 2008). "Collaboration among sponge species increases sponge diversity and abundance in a seagrass meadow". Marine Ecology. 29 (2): 193–204. Bibcode:2008MarEc..29..193W. doi:10.1111/j.1439-0485.2008.00224.x.
- ^ Duffy, J. E. (1996). "Species boundaries, specialization, and the radiation of sponge-dwelling alpheid shrimp". Biological Journal of the Linnean Society. 58 (3): 307–324. doi:10.1111/j.1095-8312.1996.tb01437.x.
- ^ Murphy, R.C. (2002). Coral Reefs: Cities Under The Seas. The Darwin Press, Inc. p. 51. ISBN 978-0-87850-138-0.
- ^ Sandford, F. (2003). "Population dynamics and epibiont associations of hermit crabs (Crustacea: Decapoda: Paguroidea) on Dog Island, Florida" (PDF). Memoirs of Museum Victoria. 60 (1): 45–52. doi:10.24199/j.mmv.2003.60.6. ISSN 1447-2554. S2CID 86167606. Archived (PDF) from the original on 2018-07-19. Retrieved 2022-01-24.
- ^ Łukowiak, M. (18 December 2020). "Utilizing sponge spicules in taxonomic, ecological and environmental reconstructions: a review". PeerJ. 8: e10601. doi:10.7717/peerj.10601. PMC 7751429. PMID 33384908.
- ^ Archer, Stephanie K.; Kahn, Amanda S.; Thiess, Mary; Law, Lauren; Leys, Sally P.; Johannessen, Sophia C.; Layman, Craig A.; Burke, Lily; Dunham, Anya (24 September 2020). "Foundation Species Abundance Influences Food Web Topology on Glass Sponge Reefs". Frontiers in Marine Science. 7. Frontiers Media SA. doi:10.3389/fmars.2020.549478. ISSN 2296-7745.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License Archived 2017-10-16 at the Wayback Machine.
- ^ a b c d Rix, L.; de Goeij, J.M.; van Oevelen, D.; Struck, U.; Al-Horani, F.A.; Wild, C.; Naumann, M.S. (23 February 2018). "Reef sponges facilitate the transfer of coral-derived organic matter to their associated fauna via the sponge loop". Marine Ecology Progress Series. 589: 85–96. Bibcode:2018MEPS..589...85R. doi:10.3354/meps12443. ISSN 0171-8630.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License Archived 2017-10-16 at the Wayback Machine
- ^ Rix L, de Goeij JM, Mueller CE, Struck U and others (2016) "Coral mucus fuels the sponge loop in warm- and coldwater coral reef ecosystems". Sci Rep, 6: 18715.
- ^ a b Rix, Laura; de Goeij, Jasper M.; van Oevelen, Dick; Struck, Ulrich; Al-Horani, Fuad A.; Wild, Christian; Naumann, Malik S. (March 2017). "Differential recycling of coral and algal dissolved organic matter via the sponge loop". Functional Ecology. 31 (3): 778−789. Bibcode:2017FuEco..31..778R. doi:10.1111/1365-2435.12758.
- ^ Crossland, C.J. (July 1987). "In situ release of mucus and DOC-lipid from the corals Acropora variabilis and Stylophora pistillata in different light regimes". Coral Reefs. 6 (1): 35−42. Bibcode:1987CorRe...6...35C. doi:10.1007/BF00302210.
- ^ a b Wild, Christian; Huettel, Markus; Klueter, Anke; Kremb, Stephan G.; Rasheed, Mohammed Y. M.; Jørgensen, Bo B. (2004). "Coral mucus functions as an energy carrier and particle trap in the reef ecosystem". Nature. 428 (6978): 66–70. Bibcode:2004Natur.428...66W. doi:10.1038/nature02344. ISSN 0028-0836. PMID 14999280.
- ^ Tanaka, Yasuaki; Miyajima, Toshihiro; Umezawa, Yu; Hayashibara, Takeshi; Ogawa, Hiroshi; Koike, Isao (2009). "Net release of dissolved organic matter by the scleractinian coral Acropora pulchra". Journal of Experimental Marine Biology and Ecology. 377 (2): 101–106. Bibcode:2009JEMBE.377..101T. doi:10.1016/j.jembe.2009.06.023.
- ^ Naumann, M. S.; Haas, A.; Struck, U.; Mayr, C.; el-Zibdah, M.; Wild, C. (September 2010). "Organic matter release by dominant hermatypic corals of the Northern Red Sea". Coral Reefs. 29 (3): 649−659. Bibcode:2010CorRe..29..649N. doi:10.1007/s00338-010-0612-7.
- ^ a b Hoegh-Guldberg, O.; McCloskey, L. R.; Muscatine, L. (April 1987). "Expulsion of zooxanthellae by symbiotic cnidarians from the Red Sea". Coral Reefs. 5 (4): 201−204. Bibcode:1987CorRe...5..201H. doi:10.1007/BF00300964.
- ^ Baghdasarian, G; Muscatine, L (2000). "Preferential expulsion of dividing algal cells as a mechanism for regulating algal-cnidarian symbiosis". The Biological Bulletin. 199 (3): 278–286. doi:10.2307/1543184. ISSN 0006-3185. JSTOR 1543184. PMID 11147708.
- ^ Crossland, C. J.; Barnes, D. J.; Borowitzka, M. A. (1980). "Diurnal lipid and mucus production in the staghorn coral Acropora acuminata". Marine Biology. 60 (2–3): 81–90. Bibcode:1980MarBi..60...81C. doi:10.1007/BF00389151. ISSN 0025-3162.
- ^ Tremblay, Pascale; Grover, Renaud; Maguer, Jean François; Legendre, Louis; Ferrier-Pagès, Christine (15 April 2012). "Autotrophic carbon budget in coral tissue: a new 13C-based model of photosynthate translocation". Journal of Experimental Biology. 215 (8): 1384–1393. doi:10.1242/jeb.065201. ISSN 1477-9145. PMID 22442377.
- ^ Ferrier-Pagès, C; Leclercq, N; Jaubert, J; Pelegrí, Sp (2000). "Enhancement of pico- and nanoplankton growth by coral exudates". Aquatic Microbial Ecology. 21: 203–209. doi:10.3354/ame021203. ISSN 0948-3055.
- ^ Wild, C.; et al. (July 2010). "Organic matter release by Red Sea coral reef organisms—potential effects on microbial activity and in situ O2 availability". Marine Ecology Progress Series. 411: 61−71. Bibcode:2010MEPS..411...61W. doi:10.3354/meps08653.
- ^ Tanaka, Yasuaki; Ogawa, Hiroshi; Miyajima, Toshihiro (2011). "Production and bacterial decomposition of dissolved organic matter in a fringing coral reef". Journal of Oceanography. 67 (4): 427–437. Bibcode:2011JOce...67..427T. doi:10.1007/s10872-011-0046-z. ISSN 0916-8370.
- ^ de Goeij, Jasper M.; van Oevelen, Dick; Vermeij, Mark J. A.; Osinga, Ronald; Middelburg, Jack J.; de Goeij, Anton F. P. M.; Admiraal, Wim (4 October 2013). "Surviving in a Marine Desert: The Sponge Loop Retains Resources Within Coral Reefs". Science. 342 (6154): 108–110. Bibcode:2013Sci...342..108D. doi:10.1126/science.1241981. ISSN 0036-8075.
- ^ Pita, L.; Rix, L.; Slaby, B. M.; Franke, A.; Hentschel, U. (2018). "The sponge holobiont in a changing ocean: from microbes to ecosystems". Microbiome. 6 (1): 46. doi:10.1186/s40168-018-0428-1. ISSN 2049-2618. PMC 5845141. PMID 29523192.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License Archived 2017-10-16 at the Wayback Machine.
- ^ Egan, Suhelen; Thomas, Torsten (16 June 2015). "Editorial for: Microbial symbiosis of marine sessile hosts- diversity and function". Frontiers in Microbiology. 6: 585. doi:10.3389/fmicb.2015.00585. ISSN 1664-302X. PMC 4468920. PMID 26136729.
- ^ a b Webster, N.S.; Thomas, T. (April 2016). "The Sponge Hologenome". mBio. 7 (2): e00135-16. doi:10.1128/mBio.00135-16. PMC 4850255. PMID 27103626.
- ^ "Spongia Linnaeus, 1759". World Register of Marine Species. Archived from the original on 27 March 2016. Retrieved 18 July 2012.
- ^ a b c Rowland, S.M.; Stephens, T. (2001). "Archaeocyatha: A history of phylogenetic interpretation". Journal of Paleontology. 75 (6): 1065–1078. doi:10.1666/0022-3360(2001)075<1065:AAHOPI>2.0.CO;2. JSTOR 1307076.
- ^ Hartman, W.D.; Goreau, T.F. (1970). "Jamaican coralline sponges: Their morphology, ecology and fossil relatives". Symposium of the Zoological Society of London. 25: 205–243. (cited by MGG.rsmas.miami.edu). Archived 2018-08-18 at the Wayback Machine
- ^ Vacelet, J. (1985). "Coralline sponges and the evolution of the Porifera". In Conway Morris, S.; George, J.D.; Gibson, R.; Platt, H.M. (eds.). The Origins and Relationships of Lower Invertebrates. Oxford University Press. pp. 1–13. ISBN 978-0-19-857181-0.
- ^ a b c Gazave, Eve; Lapébie, Pascal; Renard, Emmanuelle; Vacelet, Jean; Rocher, Caroline; Ereskovsky, Alexander V.; Lavrov, Dennis V.; Borchiellini, Carole (December 2010). "Molecular phylogeny restores the supra-generic subdivision of homoscleromorph sponges (Porifera, Homoscleromorpha)". PLOS ONE. 5 (12): e14290. Bibcode:2010PLoSO...514290G. doi:10.1371/journal.pone.0014290. PMC 3001884. PMID 21179486.
- ^ a b Gazave, Eve; Lapébie, Pascal; Ereskovsky, Alexander V.; Vacelet, Jean; Renard, Emmanuelle; Cárdenas, Paco; Borchiellini, Carole (May 2012). "No longer Demospongiae: Homoscleromorpha formal nomination as a fourth class of Porifera" (PDF). Hydrobiologia. 687 (1): 3–10. Bibcode:2012HyBio.687....3G. doi:10.1007/s10750-011-0842-x. S2CID 14468684. Archived (PDF) from the original on 2019-08-01. Retrieved 2019-08-01.
- ^ Cavalcanti, F.F.; Klautau, M. (2011). "Solenoid: a new aquiferous system to Porifera". Zoomorphology. 130 (4): 255–260. doi:10.1007/s00435-011-0139-7. S2CID 21745242.
- ^ Giribet, Gonzalo; Edgecombe, Gregory (3 March 2020). The Invertebrate Tree of Life. Princeton University Press.
- ^ Schultz, Darrin; et al. (17 May 2023). "Ancient gene linkages support ctenophores as sister to other animals". Nature. 618 (7963): 110–117. Bibcode:2023Natur.618..110S. doi:10.1038/s41586-023-05936-6. PMID 37198475.
- ^ a b c Sperling, Erik; Peterson, Kevin; Pisani, David (October 2009). "Phylogenetic-Signal Dissection of Nuclear Housekeeping Genes Supports the Paraphyly of Sponges and the Monophyly of Eumetazoa". Oxford Academic. 26 (10): 2261–2274. doi:10.1093/molbev/msp148. PMID 19597161.
- ^ Borchiellini (1 January 2001). "Sponge paraphyly and the origin of Metazoa". Oxford Academic. 14 (1): 171–179. doi:10.1046/j.1420-9101.2001.00244.x. PMID 29280585.
- ^ Sperling, E. A.; Robinson, J. M.; Pisani, D.; Peterson, K. J. (January 2010). "Where's the glass? Biomarkers, molecular clocks, and microRNAs suggest a 200 Myr missing Precambrian fossil record of siliceous sponge spicules". Geobiology. 8 (1): 24–36. Bibcode:2010Gbio....8...24S. doi:10.1111/j.1472-4669.2009.00225.x. PMID 19929965. S2CID 41195363.
- ^ Reitner, J; Wörheide, G. (2002). "Non-lithistid fossil Demospongiae – origins of their palaeobiodiversity and highlights in history of preservation". In Hooper, J.N.; Van Soest, R.W. (eds.). Systema Porifera: A Guide to the Classification of Sponges (PDF). New York, NY: Kluwer Academic Plenum. Archived (PDF) from the original on 2008-12-16. Retrieved November 4, 2008.
- ^ Li, Chia-Wei; Chen, Jun-Yuan; Hua, Tzu-En (February 1998). "Precambrian sponges with cellular structures". Science. 279 (5352): 879–882. Bibcode:1998Sci...279..879L. doi:10.1126/science.279.5352.879. PMID 9452391.
- ^ a b c Müller, W. E. G.; Jinhe Li; Schröder, H. C.; Li Qiao; Xiaohong Wang (2007). "The unique skeleton of siliceous sponges (Porifera; Hexactinellida and Demospongiae) that evolved first from the Urmetazoa during the Proterozoic: a review". Biogeosciences. 4 (2): 219–232. Bibcode:2007BGeo....4..219M. doi:10.5194/bg-4-219-2007.
- ^ McMenamin, M.A. (2008). "Early Cambrian sponge spicules from the Cerro Clemente and Cerro Rajón, Sonora, México". Geologica Acta. 6 (4): 363–367.
- ^ a b Li, Chia-Wei; Chen, Jun-Yuan; Hua, Tzu-En (February 1998). "Precambrian sponges with cellular structures". Science. 279 (5352): 879–82. Bibcode:1998Sci...279..879L. doi:10.1126/science.279.5352.879. PMID 9452391. S2CID 38837724.
- ^ Turner, E.C. (August 2021). "Possible poriferan body fossils in early Neoproterozoic microbial reefs". Nature. 596 (7870): 87–91. Bibcode:2021Natur.596...87T. doi:10.1038/s41586-021-03773-z. PMC 8338550. PMID 34321662.
- ^ "Demospongia". University of California Museum of Paleontology. Berkeley, CA: U.C. Berkeley. Archived from the original on October 18, 2013. Retrieved 2008-11-27.
- ^ Vinn, Olev; Wilson, Mark A.; Toom, Ursula; Mõtus, Mari-Ann (2015). "Earliest known rugosan-stromatoporoid symbiosis from the Llandovery of Estonia (Baltica)". Palaeogeography, Palaeoclimatology, Palaeoecology. 31: 1–5. Bibcode:2015PPP...431....1V. doi:10.1016/j.palaeo.2015.04.023. Archived from the original on 2024-04-10. Retrieved 2015-06-18.
- ^ Kodner, Robin B.; Summons, Roger E.; Pearson, Ann; King, Nicole; Knoll, Andrew H. (July 2008). "Sterols in a unicellular relative of the metazoans". Proceedings of the National Academy of Sciences of the United States of America. 105 (29): 9897–9902. Bibcode:2008PNAS..105.9897K. doi:10.1073/pnas.0803975105. PMC 2481317. PMID 18632573.
- ^ Nichols, S.; Wörheide, G. (April 2005). "Sponges: New views of old animals". Integrative and Comparative Biology. 45 (2): 333–334. CiteSeerX 10.1.1.598.4999. doi:10.1093/icb/45.2.333. PMID 21676777.
- ^ Love, Gordon D.; Grosjean, Emmanuelle; Stalvies, Charlotte; Fike, David A.; Grotzinger, John P.; Bradley, Alexander S.; et al. (February 2009). "Fossil steroids record the appearance of Demospongiae during the Cryogenian period" (PDF). Nature. 457 (7230): 718–721. Bibcode:2009Natur.457..718L. doi:10.1038/nature07673. PMID 19194449. S2CID 4314662. Archived from the original (PDF) on 2018-07-24. Retrieved 2019-08-01.
- ^ Antcliffe, J.B. (2013). Stouge, S. (ed.). "Questioning the evidence of organic compounds called sponge biomarkers". Palaeontology. 56 (5): 917–925. Bibcode:2013Palgy..56..917A. doi:10.1111/pala.12030.
- ^ Gold, D.A. (September 2018). "The slow rise of complex life as revealed through biomarker genetics". Emerging Topics in Life Sciences. 2 (2): 191–199. doi:10.1042/ETLS20170150. PMID 32412622. S2CID 90887224.
- ^ Gold, David A.; Grabenstatter, Jonathan; de Mendoza, Alex; Riesgo, Ana; Ruiz-Trillo, Iñaki; Summons, Roger E. (March 2016). "Sterol and genomic analyses validate the sponge biomarker hypothesis". Proceedings of the National Academy of Sciences of the United States of America. 113 (10): 2684–2689. Bibcode:2016PNAS..113.2684G. doi:10.1073/pnas.1512614113. PMC 4790988. PMID 26903629.
- ^ a b Porter, S.M. (2008). "Skeletal microstructure indicates Chancelloriids and Halkieriids are closely related". Palaeontology. 51 (4): 865–879. Bibcode:2008Palgy..51..865P. doi:10.1111/j.1475-4983.2008.00792.x.
- ^ Butterfield, N.J.; Nicholas, C.J. (1996). "Burgess Shale-type preservation of both non-mineralizing and "shelly" Cambrian organisms from the Mackenzie Mountains, northwestern Canada". Journal of Paleontology. 70 (6): 893–899. Bibcode:1996JPal...70..893B. doi:10.1017/S0022336000038579. JSTOR 1306492. S2CID 133427906.
- ^ Janussen, Dorte; Steiner, Michael; Maoyan, Zhu (2002). "New well-preserved scleritomes of Chancelloridae from the early Cambrian Yuanshan Formation (Chengjiang, China) and the middle Cambrian Wheeler Shale (Utah, USA) and paleobiological implications". Journal of Paleontology. 76 (4): 596–606. Bibcode:2002JPal...76..596J. doi:10.1666/0022-3360(2002)076<0596:NWPSOC>2.0.CO;2. S2CID 129127213. free text at Janussen, D. (2002). "(full text without images)". Journal of Paleontology. Archived from the original on December 10, 2008. Retrieved 2008-08-04.
- ^ a b c Borchiellini, C.; Manuel, M.; Alivon, E.; Boury-Esnault, N.; Vacelet, J.; Le Parco, Y. (January 2001). "Sponge paraphyly and the origin of Metazoa". Journal of Evolutionary Biology. 14 (1): 171–179. doi:10.1046/j.1420-9101.2001.00244.x. PMID 29280585.
- ^ a b Sperling, E. A.; Pisani, D.; Peterson, K. J. (2007). "Poriferan paraphyly and its implications for Precambrian paleobiology" (PDF). Journal of the Geological Society of London. 286 (1): 355–368. Bibcode:2007GSLSP.286..355S. doi:10.1144/SP286.25. S2CID 34175521. Archived from the original (PDF) on May 9, 2009. Retrieved 2008-11-04.
- ^ Medina, Mónica; Collins, Allen G.; Silberman, Jeffrey D.; Sogin, Mitchell L. (August 2001). "Evaluating hypotheses of basal animal phylogeny using complete sequences of large and small subunit rRNA". Proceedings of the National Academy of Sciences of the United States of America. 98 (17): 9707–12. Bibcode:2001PNAS...98.9707M. doi:10.1073/pnas.171316998. PMC 55517. PMID 11504944.
- ^ a b Dunn, Casey W.; Hejnol, Andreas; Matus, David Q.; Pang, Kevin; Browne, William E.; Smith, Stephen A.; et al. (April 2008). "Broad phylogenomic sampling improves resolution of the animal tree of life". Nature. 452 (7188): 745–9. Bibcode:2008Natur.452..745D. doi:10.1038/nature06614. PMID 18322464. S2CID 4397099.
- ^ Hejnol, Andreas; Obst, Matthias; Stamatakis, Alexandros; Ott, Michael; Rouse, Greg W.; Edgecombe, Gregory D.; et al. (December 2009). "Assessing the root of bilaterian animals with scalable phylogenomic methods". Proceedings. Biological Sciences. 276 (1677): 4261–70. doi:10.1098/rspb.2009.0896. PMC 2817096. PMID 19759036.
- ^ Ryan, Joseph F.; Pang, Kevin; Schnitzler, Christine E.; Nguyen, Anh-Dao; Moreland, R. Travis; Simmons, David K.; et al. (December 2013). "The genome of the ctenophore Mnemiopsis leidyi and its implications for cell type evolution". Science. 342 (6164): 1242592. doi:10.1126/science.1242592. PMC 3920664. PMID 24337300.
- ^ Moroz, Leonid L.; Kocot, Kevin M.; Citarella, Mathew R.; Dosung, Sohn; Norekian, Tigran P.; Povolotskaya, Inna S.; et al. (2014). "The ctenophore genome and the evolutionary origins of neural systems". Nature. 510 (7503): 109–114. Bibcode:2014Natur.510..109M. doi:10.1038/nature13400. ISSN 0028-0836. PMC 4337882. PMID 24847885.
- ^ Pisani, Davide; Pett, Walker; Dohrmann, Martin; Feuda, Roberto; Rota-Stabelli, Omar; Philippe, Hervé; Lartillot, Nicolas; Wörheide, Gert (December 2015). "Genomic data do not support comb jellies as the sister group to all other animals". Proceedings of the National Academy of Sciences of the United States of America. 112 (50): 15402–15407. Bibcode:2015PNAS..11215402P. doi:10.1073/pnas.1518127112. PMC 4687580. PMID 26621703.
- ^ Berwald, Juli (2017). Spineless: the science of jellyfish and the art of growing a backbone. Riverhead Books. ISBN 9780735211261.[page needed]
- ^ Schierwater, Bernd; Eitel, Michael; Jakob, Wolfgang; Osigus, Hans-Jürgen; Hadrys, Heike; Dellaporta, Stephen L.; Kolokotronis, Sergios-Orestis; DeSalle, Rob (January 2009). "Concatenated analysis sheds light on early metazoan evolution and fuels a modern "urmetazoon" hypothesis". PLOS Biology. 7 (1): e20. doi:10.1371/journal.pbio.1000020. PMC 2631068. PMID 19175291.
- ^ Kapli, P.; Telford, M.J. (December 2020). "Topology-dependent asymmetry in systematic errors affects phylogenetic placement of Ctenophora and Xenacoelomorpha". Science Advances. 6 (50): eabc5162. Bibcode:2020SciA....6.5162K. doi:10.1126/sciadv.abc5162. PMC 7732190. PMID 33310849.
- ^ Simion, Paul; Philippe, Hervé; Baurain, Denis; Jager, Muriel; Richter, Daniel J.; Di Franco, Arnaud; et al. (April 2017). "A Large and Consistent Phylogenomic Dataset Supports Sponges as the Sister Group to All Other Animals" (PDF). Current Biology. 27 (7): 958–967. Bibcode:2017CBio...27..958S. doi:10.1016/j.cub.2017.02.031. PMID 28318975. Archived (PDF) from the original on 2020-04-25. Retrieved 2018-11-04.
- ^ Redmond, A.K.; McLysaght, A. (March 2021). "Evidence for sponges as sister to all other animals from partitioned phylogenomics with mixture models and recoding". Nature Communications. 12 (1): 1783. Bibcode:2021NatCo..12.1783R. doi:10.1038/s41467-021-22074-7. PMC 7979703. PMID 33741994.
- ^ Schultz, Darrin T.; Haddock, Steven H. D.; Bredeson, Jessen V.; Green, Richard E.; Simakov, Oleg; Rokhsar, Daniel S. (June 2023). "Ancient gene linkages support ctenophores as sister to other animals". Nature. 618 (7963): 110–117. Bibcode:2023Natur.618..110S. doi:10.1038/s41586-023-05936-6. PMC 10232365. PMID 37198475.
- ^ Smolker, Rachel; Richards, Andrew; Connor, Richard; Mann, Janet; Berggren, Per (1997). "Sponge-carrying by Indian Ocean bottlenose dolphins: Possible tool-use by a delphinid". Ethology. 103 (6): 454–465. doi:10.1111/j.1439-0310.1997.tb00160.x. hdl:2027.42/71936.
- ^ McClenachan, L. (2008). "Social conflict, Over-fishing and Disease in the Florida Sponge Fishery, 1849–1939". In Starkey, D.J.; Holm, P.; Barnard, M. (eds.). Oceans Past: Management Insights from the History of Marine Animal Populations. Earthscan. pp. 25–27. ISBN 978-1-84407-527-0.
- ^ Jacobson, N. (2000). Cleavage. Rutgers University Press. p. 62. ISBN 978-0-8135-2715-4.
- ^ "Sponges". Cervical Barrier Advancement Society. 2004. Archived from the original on January 14, 2009. Retrieved 2006-09-17.
- ^ Porterfield, W.M. (1955). "Loofah — The sponge gourd". Economic Botany. 9 (3): 211–223. Bibcode:1955EcBot...9..211P. doi:10.1007/BF02859814. S2CID 27313678.
- ^ Imhoff, J.F.; Stöhr, R. (2003). "Sponge-Associated Bacteria". In Müller, W.E. (ed.). Sponges (Porifera): Porifera. Springer. pp. 43–44. ISBN 978-3-540-00968-9.
- ^ Teeyapant, R.; Woerdenbag, H. J.; Kreis, P.; Hacker, J.; Wray, V.; Witte, L.; Proksch, P. (1993). "Antibiotic and cytotoxic activity of brominated compounds from the marine sponge Verongia aerophoba". Zeitschrift für Naturforschung C. 48 (11–12): 939–45. doi:10.1515/znc-1993-11-1218. PMID 8297426. S2CID 1593418.
- ^ Takeuchi, Shinji; Ishibashi, Masami; Kobayashi, Junichi (1994). "Plakoridine A, a new tyramine-containing pyrrolidine alkaloid from the Okinawan marine sponge Plakortis sp". Journal of Organic Chemistry. 59 (13): 3712–3713. doi:10.1021/jo00092a039.
- ^ Etchells, Laura L.; Sardarian, Ali; Whitehead, Roger C. (18 April 2005). "A synthetic approach to the plakoridines modeled on a biogenetic theory". Tetrahedron Letters. 46 (16): 2803–2807. doi:10.1016/j.tetlet.2005.02.124.
Further reading
- Hickman, C.; Roberts, L.; Larson, A. (2003). Animal Diversity (3rd ed.). New York: McGraw-Hill. ISBN 978-0-07-234903-0.
- Ereskovsky, A.V. (2010). The Comparative Embryology of Sponges. Russia: Springer Science+Business Media. ISBN 978-90-481-8575-7.
- Wörheide, G. (April 2008). "A hypercalcified sponge with soft relatives: Vaceletia is a keratose demosponge". Molecular Phylogenetics and Evolution. 47 (1): 433–8. Bibcode:2008MolPE..47..433W. doi:10.1016/j.ympev.2008.01.021. PMID 18321733.
External links
- Water flow and feeding in the phylum Porifera (sponges) – Flash animations of sponge body structures, water flow and feeding
- Carsten's Spongepage, Information on the ecology and the biotechnological potential of sponges and their associated bacteria.
- History of Tarpon Springs sponge industry, Tarpon Springs, Florida
- Nature's 'fibre optics' experts
- The Sponge Reef Project
- Queensland Museum information about sponges
- Queensland Museum Sessile marine invertebrates collections
- Queensland Museum Sessile marine invertebrates research
- Sponge Guide for Britain and Ireland Archived 2008-12-08 at the Wayback Machine, Bernard Picton, Christine Morrow & Rob van Soest
- World Porifera database, the world list of extant sponges, includes a searchable database.
- Sponges: World production and markets // Food and Agriculture Organisation




