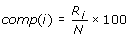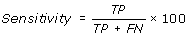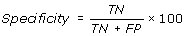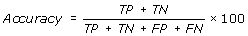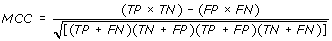Identification of conformational B-cell Epitopes in an antigen from its primary sequence
- Research
- Open Access
Identification of conformational B-cell Epitopes in an antigen from its primary sequence
- Hifzur Rahman Ansari1_44 and
- Gajendra PS Raghava1_44Email author
- Received: 20 May 2010
- Accepted: 20 October 2010
- Published: 20 October 2010
Abstract
Background
One of the major challenges in the field of vaccine design is to predict conformational B-cell epitopes in an antigen. In the past, several methods have been developed for predicting conformational B-cell epitopes in an antigen from its tertiary structure. This is the first attempt in this area to predict conformational B-cell epitope in an antigen from its amino acid sequence.
Results
All Support vector machine (SVM) models were trained and tested on 187 non-redundant protein chains consisting of 2261 antibody interacting residues of B-cell epitopes. Models have been developed using binary profile of pattern (BPP) and physiochemical profile of patterns (PPP) and achieved a maximum MCC of 0.22 and 0.17 respectively. In this study, for the first time SVM model has been developed using composition profile of patterns (CPP) and achieved a maximum MCC of 0.73 with accuracy 86.59%. We compare our CPP based model with existing structure based methods and observed that our sequence based model is as good as structure based methods.
Conclusion
This study demonstrates that prediction of conformational B-cell epitope in an antigen is possible from is primary sequence. This study will be very useful in predicting conformational B-cell epitopes in antigens whose tertiary structures are not available. A web server CBTOPE has been developed for predicting B-cell epitope http://www.imtech.res.in/raghava/cbtope/.
Keywords
- Support Vector Machine
- Area Under Curve
- Support Vector Machine Model
- Window Length
- Benchmark Dataset
Background
A region or segment of an antigen, recognized by a specific antibody or B-cell is called antigenic region or B-cell epitope. These B-cell epitopes can be categorized into two classes, continuous and discontinuous. A continuous/linear epitope is a segment of consecutive residues in the primary sequence while a discontinuous/conformational epitope is a bunch of residues of an antigen that are far away from each other in the primary sequence but are brought to spatial proximity as a result of polypeptide folding. It is also known that most of the B-cell epitope (~90%) are conformational epitope. Both types of epitopes play an important role in the peptide-based vaccines and disease diagnosis [1, 2]. One of the beauties of immune system is that it recognizes the foreign proteins/antigens and generate specific antibody against these antigens. This potential of immune system has been exploited by researchers for designing subunit vaccines [3, 4].
In the post genomic era where a large number of pathogens have been completely sequenced, it is crucial to identify B-cell epitope or here after called antibody interacting residues in an antigen for the design of subunit vaccines against these pathogens. In the past several experimental techniques have been developed for mapping antibody interacting residues on an antigen that includes identification of interacting residues from structure of antibody-antigen complexes [5]. One of the popular approaches is overlapping peptide synthesis covering the entire antigen sequence, which identifies mainly sequential epitopes [6]. Mapping of antibody interacting residues has been severely hampered by the costly and time taking process of 3D structure determination. Many tools, covering compilation, visualization and prediction of B and T cell epitopes have been developed [7]. Despite of majority of epitopes being conformational, most of the computational methods and databases centered at the sequential epitopes [8, 9, 10]. Linear epitope prediction methods can be categorized into physico-chemical property [11], HMM [12] and ANN based [13]. Many methods are available for antibody interacting residues identification if antigen's or its homolog's tertiary structure is known which in itself is a big limitation. These are based on features like flexibility, solvent accessibility [14, 15] and amino acid propensity scales [16]. Earlier researchers created a benchmark dataset from the 3D PDB structures and evaluated several structure-based protein-protein binding site prediction methods which included popular CEP [15] and DiscoTope [16] for predicting immunogenic regions [17]. They opted the definition, that epitope consist of antigen residues in which any atom of the antigen residue is separated from any antibody atom by a distance of ≤ 4Å. They found that the performance of all methods were mediocre and no method could achieve Area under curve (AUC) greater than 0.7. In addition to these a bunch of improved methods have been developed for the prediction of antibody interacting residues if tertiary structure of antigen is known [18, 19, 20, 21, 22, 23]. In summary, one needs to determine structure of antigen using crystallography in order to identify antibody interacting residues in antigen. The experimental techniques like crystallography are expensive and time consuming where as functional assays are not reliable enough [5]. Thus there is need to develop alternate technique for predicting antibody interacting residues in a protein.
In this study attempt has been made to predict antibody interacting residues in an antigen from its primary sequence. First we created the patterns of different window lengths from the corresponding amino acid sequences then used the standard binary and physico-chemical profiles of patterns. We have introduced for the first time the concept of composition profile of pattern (CPP) generated through sliding window where the central residue is antibody interacting. These features were used to develop SVM based models to predict antibody interacting residues with high accuracy.
Methods
Definition of antibody interacting residues or epitope
There are many levels of antigen-antibody interactions one can obtain from PDB structures. Among these interactions we defined antibody interacting residue as a residue of antigen which is at least one atom separated from an antibody atom by 4Å distance. We borrowed this definition from benchmark paper [17] in order to compare our models with existing methods.
Datasets
Main dataset
We obtained 526 antigenic sequences combined from IEDB database and benchmark dataset [9, 17]. Sequence redundancy was removed using program CDHIT [24] at 40% cutoff. Finally we got 187 antigens where no two sequences have more than 40% sequence identity. These antigens have 2261 antibody interacting or 2261 residues are part of conformational B-cell epitope and 107414 amino acid residues were non-antibody interacting from the same antigen sequences.
Benchmark Dataset
In addition to main dataset, we also evaluate our models on benchmark dataset [17] which contains 161 protein chains from 144 antigen-antibody complex structures. Finally we got non-redundant set of 52 antigen chains where no two sequences have more than 40% sequence identity. This benchmark dataset of 52 antigens contains 858 antibody interacting and 9366 non-antibody interacting residues.
Creation of patterns
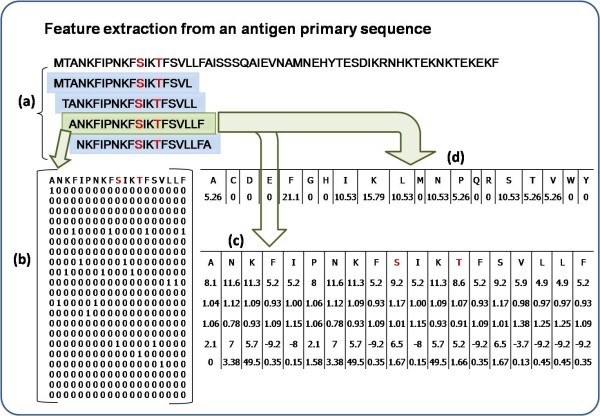
Feature extraction for a 19 window length pattern. Antibody interacting residues are marked in red e.g. S/T, Positive pattern shaded in green where S is at the center with 9 neighboring residues on either side, other overlapping negative patterns are shown in blue. a) Creation of 19 window overlapping patterns from amino acid sequence, b) generation of binary profile of pattern (BPP), c) generation of physico-chemical profile (PPP) and d) generation of composition profile of pattern (CPP).
Realistic and balance learning
In order to develop prediction method one needs to generate overlapping patterns for each antigen in a dataset; one pattern for each residue. It will produce two types of patterns positive and negative, positive patterns have antibody interacting central residue. These patterns are used to train machine-learning techniques for developing models. In real life only few residues in an antigen are recognized by antibody or B-cell receptor. This means that the number of negative patterns will be much higher than positive patterns in our training dataset; for 2261 positive patterns there were 107414 negative patterns. This creates two problems; i) poor performance of models due to imbalanced set of patterns and ii) training of models is time consuming and CPU intensive. Thus in this study we have used two pattern sets for learning our models; i) realistic set of patterns that includes all negative patterns and ii) balance set of patterns having equal number of positive and negative patterns. In case of balance set, we randomly picked up equal number of negatives from negative pattern set.
Derivation of features from patterns
Binary profile of patterns (BPP)
Each pattern was converted into binary profile, where an amino acid was represented by a vector of dimension 21 (e.g. Ala by 1,0,0,0,0,0,0,0,0,0,0,0,0,0,0,0,0,0,0,0,0). A pattern of window length W was represented by a vector of dimensions 21xW (Additional file 1, Table S1). The binary profile has been used in a number of existing methods [30, 31].
Physico-chemical profile of patterns (PPP)
As amino acids' physico-chemical properties contribute in the determination of its structure and function, we selected five properties tested by others [32]. These are Grantham polarity [33], Karplus-Schulz flexibility [34], Kolaskar antigencity [35], Parker hydrophobicity [36] and Ponnuswami polarity index [37]. Physico-chemical profile of patterns is similar to the BPP, the only difference lies in the properties of amino acids. Here each amino acid is represented by a vector of 5 i.e. each pattern converted into a vector size of 5xW. For example Ala is represented as [pHydrophobicity, pFlexibility, pPolarity_Grantham, pPolarity_Ponnuswami, pAntigenecity] corresponding to different property values (Additional file 1, Table S2).
Composition profile of patterns (CPP)
Where comp (i) is the percent composition of a residue of type i; Ri is number of residues of type i, and N is the total the number of residues in the pattern.
Support Vector Machines (SVM)
In the past SVM had been used in a number of biological problems, from classification to functional prediction of proteins [40, 41, 42]. In the present study, we have developed a SVM model using a powerful package SVM_light http://svmlight.joachims.org/, for predicting antibody interacting residues in proteins.
Cross-validation technique
There are many techniques for evaluating the performance of models like leave-one-out or jack-knife test, n-fold cross validation etc [43]. Though jackknife test is the best among cross-validation techniques [44], it is time consuming and CPU intensive technique [40, 45]. In order to save time and resources we used widely acceptable 5-fold cross-validation technique. In this technique data is randomly divided into five equal sets of which four sets are used for training and the remaining fifth set for testing. This process is repeated five times in such a way that each set is used once for testing. Final performance is the average of performances achieved on the five sets.
Performance Measures
[TP = true positive; FN = false negative; TN = true negative; FP = false positive]
We created ROC (receiver operating curve) for all of the models in order to evaluate performance of models using threshold independent parameters. ROC plots with Area under curve (AUC) were created using SPSS statistical package.
Results
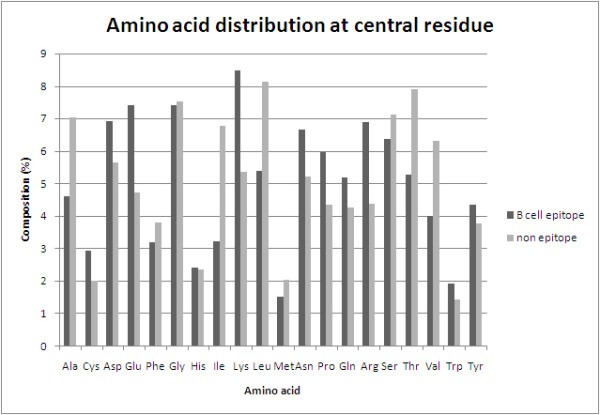
Analysis of antibody interacting residues
Comparison of amino acid composition of antibody interacting residues (B-cell epitope) and non-interacting residues (non-epitope).
SVM Models based on BPP and PPP
The performance of BPP based SVM model developed using different window lengths from 5 to 21 residues
Window size |
Kernel parameters |
Thr* |
Sen |
Spe |
Acc |
MCC |
|---|---|---|---|---|---|---|
5 |
t 2 g 0.01 j 1 c 10 |
0.1 |
58.38 |
58.55 |
58.47 |
0.17 |
7 |
t 2 g 0.01 j 1 c 1 |
0.1 |
55.87 |
59.81 |
57.84 |
0.16 |
9 |
t 2 g 0.01 j 1 c 1 |
0.1 |
55.66 |
58.85 |
57.26 |
0.15 |
11 |
t 2 g 0.001 j 1 c 10 |
0 |
61.55 |
56.99 |
59.27 |
0.19 |
13 |
t 2 g 0.1 j 1 c 1 |
0 |
62.58 |
59.09 |
60.84 |
0.22 |
15 |
t 2 g 0.1 j 1 c 10 |
0 |
59.93 |
57.63 |
58.78 |
0.18 |
17 |
t 2 g 0.001 j 1 c 10 |
0 |
58.37 |
57.18 |
57.78 |
0.16 |
19 |
t 2 g 0.001 j 1 c 10 |
0.1 |
52.92 |
63.78 |
58.35 |
0.17 |
21 |
t 2 g 0.001 j 1 c 10 |
0 |
59.69 |
57.22 |
58.45 |
0.17 |
The performance of PPP based SVM model developed different window lengths from 5 to 21 residues
W |
Kernel parameters |
Thr* |
Sen |
Spe |
Acc |
MCC |
|---|---|---|---|---|---|---|
5 |
t 2 g 0.00001 j 1 c 10 |
-0.3 |
53.95 |
59.62 |
56.78 |
0.14 |
7 |
t 2 g 0.00001 j 1 c 10 |
0.1 |
55.82 |
58.03 |
56.93 |
0.14 |
9 |
t 2 g 0.00001 j 1 c 10 |
0 |
54.56 |
55.84 |
55.2 |
0.1 |
11 |
t 2 g 0.00001 j 1 c 10 |
0.1 |
52.3 |
62.48 |
57.39 |
0.15 |
13 |
t 2 g 0.00001 j 1 c 10 |
0.1 |
55.11 |
60.37 |
57.74 |
0.16 |
15 |
t 2 g 0.00001 j 1 c 10 |
0 |
56.57 |
60.06 |
58.31 |
0.17 |
17 |
t 2 g 0.00001 j 1 c 10 |
0 |
60.19 |
55.77 |
57.98 |
0.16 |
19 |
t 2 g 0.00001 j 1 c 10 |
0 |
57.82 |
54.15 |
55.98 |
0.12 |
21 |
t 1 d 1 |
0 |
57.31 |
58.32 |
57.81 |
0.16 |
SVM Model using Composition Profile of Patterns (CPP)
The performance SVM models developed using composition profile of patterns at different window lengths
Window size |
Kernel parameters |
Thr* |
Sen |
Spe |
Acc |
MCC |
|---|---|---|---|---|---|---|
5 |
t 2 g 0.001 j 1 c 1 |
0 |
61.75 |
58.11 |
59.93 |
0.2 |
7 |
t 2 g 0.001 j 1 c 10 |
0 |
68.35 |
62.2 |
65.27 |
0.31 |
9 |
t 2 g 0.001 j 1 c 10 |
0 |
73.45 |
67.21 |
70.33 |
0.41 |
11 |
t 2 g 0.01 j 1 c 1 |
-0.1 |
82.08 |
77.26 |
79.67 |
0.59 |
13 |
t 2 g 0.01 j 1 c 10 |
-0.1 |
82.57 |
84.17 |
83.37 |
0.67 |
15 |
t 2 g 0.01 j 1 c 1 |
-0.1 |
79.96 |
90.31 |
85.14 |
0.71 |
17 |
t 2 g 0.01 j 1 c 1 |
-0.1 |
80.69 |
90.1 |
85.4 |
0.71 |
19 |
t 2 g 0.01 j 1 c 1 |
-0.1 |
83.13 |
90.06 |
86.59 |
0.73 |
21 |
t 2 g 0.01 j 1 c 1 |
-0.1 |
83.62 |
88.96 |
86.29 |
0.73 |
The performance of SVM models developed using composition, binary and physic-chemical property profile.
Comparison with existing methods
The performance of BPP and CPP based SVM model on Benchmark dataset, developed using balance and realistic set of patterns.
Type of Pattern set |
Model |
SVM parameters |
Thr* |
Sen |
Spe |
Acc |
MCC |
|---|---|---|---|---|---|---|---|
Realistic |
BPP |
t 2 g 0.001 j 10 c 10 |
-0.2 |
50.49 |
60.28 |
59.49 |
0.06 |
CPP |
t 2 g 0.001 j 10 c 10 |
-0.3 |
80.41 |
84.64 |
84.30 |
0.44 |
|
Balance |
BPP |
t 2 g 0.01 j 1 c 10 |
0.1 |
61.31 |
51.22 |
56.27 |
0.13 |
CPP |
t 2 g 0.01 j 1 c 10 |
0 |
82.36 |
89.42 |
85.89 |
0.72 |
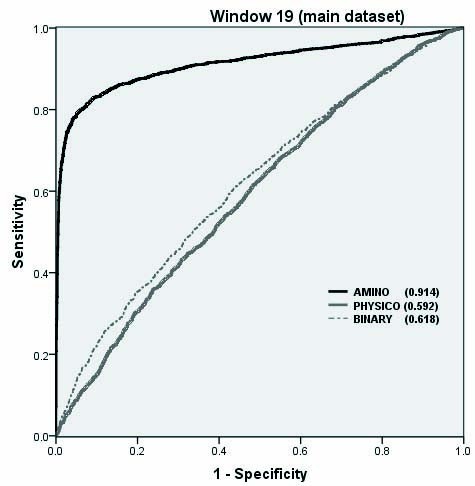
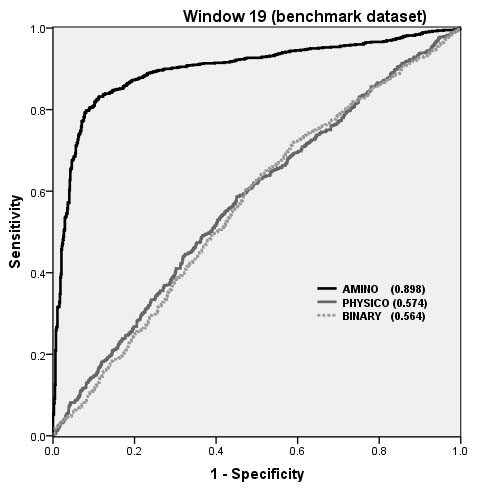
The performance of SVM models on Benchmark dataset as shown by ROC plot.
Overall performance of structure based and CBTOPE algorithms on benchmark dataset
Evaluation parameter |
ProMate |
PSI-PRED best patch |
Patch Dock best model |
ClusPro (DOT) best model |
CEP |
DiscoTope (-7.7) |
CBTOPE* (This Study) |
|---|---|---|---|---|---|---|---|
Sen* |
0.09 |
0.33 |
0.43 |
0.45 |
0.31 |
0.42 |
0.80 |
1-Spe |
0.08 |
0.14 |
0.11 |
0.07 |
0.22 |
0.21 |
0.15 |
PPV |
0.10 |
0.19 |
0.26 |
0.39 |
0.11 |
0.16 |
0.31 |
Acc |
0.84 |
0.82 |
0.85 |
0.89 |
0.74 |
0.75 |
0.84 |
AUC |
0.51 |
0.60 |
0.66 |
0.69 |
0.54 |
0.60 |
0.89 |
Implementation
A user-friendly web server 'CBTOPE' was developed for the prediction of antibody interacting residues or B-cell conformational epitopes. The server is developed using CGI-Perl script, HTML and installed on a Sun Server (420E) under UNIX (Solaris 7) environment. The user may submit the amino acid sequence(s) in 'FASTA' format. The server generates the 19 window patterns of all submitted sequences, calculates amino acid composition and predicts antibody interacting residues. The output is the amino acid sequence mapped with a probability scale ranging from 0 to 9 for each amino acid. 0 indicates the rarest chance of being that residue in a B-cell epitope and 9 as the most probable. We suggest that for high specificity (high confidence) prediction, user should select the higher threshold value but compromising the sensitivity of prediction. However, for maximum prediction of antibody interacting residues user should opt lower threshold. There is always interplay between sensitivity and specificity. The default threshold was set at -0.3 as at this value, sensitivity and specificity was found equal during the development. Web-server is freely available at http://www.imtech.res.in/raghava/cbtope.
Discussion
It has been a great challenge for the academicians to devise algorithms and methods for the identification and mapping of potential B-cell epitopes from an antigen sequence. Much effort has been put in trying to predict the conformational B-cell epitope. Previous methods predict conformational B-cell epitopes with reasonably high accuracy, the limitation of these methods is that they require tertiary structure of the antigen. Experimental technique like X-ray crystallography used for determining structure of a protein is costly, tedious and time consuming. To the best of author's knowledge there is no method which can predict conformational B-cell epitopes in an antigen in absence of tertiary structure. There is a need to develop methods for predicting conformational B-cell epitopes in an antigen from its primary sequence. This study describes the method CBTOPE developed for predicting conformational epitopes of antibody interacting residues in antigens. In order to compare performance of our models we chose a benchmark dataset, which was used to evaluate the performance of structure based methods. In order to increase the data we included data from IEDB database. We presumed that the antibody interacting residues are the conformational B-cell epitope residues. We used traditional features of binary and physico-chemical profiles of patterns, evaluated by 5-fold cross validation while using SVM as a classifier. Performance was very poor in BPP models due to the fact that for 21xW vector size only W values represent 1, the rest all are 0 so the noise is more in BPP model. PPP model also could not perform well although it was earlier used for linear and structure based conformational B-cell epitope prediction. From the preliminary analysis of the composition and 2 sample logo plots of positive and negative patterns, it was clear that there is significant difference in the composition and surface propensities of certain residues which can be exploited to discriminate the patterns. Finally we used for the first time, in our study simple amino acid composition model of patterns (CPP) with vector size of 20 which was evaluated on two different datasets. The performance improved significantly and it is interesting to note that it can be used for the prediction of conformational B-cell epitopes despite the fact that in CPP model we lost the amino acid order information unlike BPP. This problem may be equated to the sub-cellular localization of proteins wherein it was observed that simple amino acid composition model perform better than other features. But unlike sub-cellular localization we exploited composition of patterns instead of whole protein sequence. It should be noted that despite the prediction of antibody interacting or individual B-cell epitope residues, being a sequence based method and the lack of 3D structural input, CBTOPE cannot assist in determining the number and distance needed to make an epitope segment in the antigen sequence. This information can be obtained by mapping of the predicted residues on the modeled structure. We hope that the present model is unique in its kind and will compliment the available structure based methods used for the prediction of antibody interacting residues or conformational B-cell epitopes.
Conclusion
We showed that simple antigen sequence can be used for the prediction of conformational B-cell epitopes and no structure or homology is required. We introduced for the first time concept of local amino acid composition of antigen. We showed that our CPP composition based SVM model outperformed other structure methods with better sensitivity and AUC on the same benchmark dataset.
Declarations
Acknowledgements
The author's are thankful to the Council of Scientific and Industrial Research (CSIR) and Department of Biotechnology (DBT), Government of India for financial assistance. Hifzur Rahman Ansari is a Senior Research Fellow and financially supported by CSIR.
Authors’ Affiliations
References
- Gershoni JM, Roitburd-Berman A, Siman-Tov DD, Tarnovitski Freund N, Weiss Y: Epitope mapping the first step in developing epitope-based vaccines. BioDrugs 2007, 21:145–156.View ArticlePubMedGoogle Scholar
- Pomes A: Relevant B cell epitopes in allergic disease. Int Arch Allergy Immunol 2010, 152:1–11.View ArticlePubMedGoogle Scholar
- Almagro JC: Identification of differences in the specificity-determining residues of antibodies that recognize antigens of different size: implications for the rational design of antibody repertoires. J Mol Recognit 2004, 17:132–143.View ArticlePubMedGoogle Scholar
- MacCallum RM, Martin AC, Thornton JM: Antibody-antigen interactions: contact analysis and binding site topography. J Mol Biol 1996, 262:732–745.View ArticlePubMedGoogle Scholar
- Van Regenmortel MH: Structural and functional approaches to the study of protein antigenicity. Immunol Today 1989, 10:266–272.View ArticlePubMedGoogle Scholar
- Frank R: The SPOT-synthesis technique. Synthetic peptide arrays on membrane supports--principles and applications. J Immunol Methods 2002, 267:13–26.View ArticlePubMedGoogle Scholar
- Xingdong Y, Xinglong Y: An introduction to epitope prediction methods and software. Reviews in Medical Virology 2009, 19:77–96.View ArticleGoogle Scholar
- Saha S, Raghava GP: Searching and mapping of B-cell epitopes in Bcipep database. Methods Mol Biol 2007, 409:113–124.View ArticlePubMedGoogle Scholar
- Vita R, Zarebski L, Greenbaum JA, Emami H, Hoof I, Salimi N, Damle R, Sette A, Peters B: The immune epitope database 2.0. Nucleic Acids Res 2010, 38:D854–862.View ArticlePubMedGoogle Scholar
- Saha S, Raghava GP: Prediction methods for B-cell epitopes. Methods Mol Biol 2007, 409:387–394.View ArticlePubMedGoogle Scholar
- Saha S, Raghava GP: BcePred: Prediction of continuous B-cell epitopes in antigenic sequences using physico-chemical properties. ICARIS, LNCS 2004, 3239:197–204.Google Scholar
- Larsen JE, Lund O, Nielsen M: Improved method for predicting linear B-cell epitopes. Immunome Res 2006, 2:2.View ArticlePubMedGoogle Scholar
- Saha S, Raghava GP: Prediction of continuous B-cell epitopes in an antigen using recurrent neural network. Proteins 2006, 65:40–48.View ArticlePubMedGoogle Scholar
- Novotny J, Handschumacher M, Haber E, Bruccoleri RE, Carlson WB, Fanning DW, Smith JA, Rose GD: Antigenic determinants in proteins coincide with surface regions accessible to large probes (antibody domains). Proc Natl Acad Sci USA 1986, 83:226–230.View ArticlePubMedGoogle Scholar
- Kulkarni-Kale U, Bhosle S, Kolaskar AS: CEP: a conformational epitope prediction server. Nucleic Acids Res 2005, 33:W168–171.View ArticlePubMedGoogle Scholar
- Haste Andersen P, Nielsen M, Lund O: Prediction of residues in discontinuous B-cell epitopes using protein 3D structures. Protein Sci 2006, 15:2558–2567.View ArticlePubMedGoogle Scholar
- Ponomarenko JV, Bourne PE: Antibody-protein interactions: benchmark datasets and prediction tools evaluation. BMC Struct Biol 2007, 7:64.View ArticlePubMedGoogle Scholar
- Sweredoski MJ, Baldi P: PEPITO: improved discontinuous B-cell epitope prediction using multiple distance thresholds and half sphere exposure. Bioinformatics 2008, 24:1459–1460.View ArticlePubMedGoogle Scholar
- Moreau V, Fleury C, Piquer D, Nguyen C, Novali N, Villard S, Laune D, Granier C, Molina F: PEPOP: computational design of immunogenic peptides. BMC Bioinformatics 2008, 9:71.View ArticlePubMedGoogle Scholar
- Huang Y, Bao Y, Guo S, Wang Y, Zhou C, Li Y: Pep-3D-Search: a method for B-cell epitope prediction based on mimotope analysis. BMC Bioinformatics 2008, 9:538.View ArticlePubMedGoogle Scholar
- Huang J, Gutteridge A, Honda W, Kanehisa M: MIMOX: a web tool for phage display based epitope mapping. BMC Bioinformatics 2006, 7:451.View ArticlePubMedGoogle Scholar
- Bublil EM, Freund NT, Mayrose I, Penn O, Roitburd-Berman A, Rubinstein ND, Pupko T, Gershoni JM: Stepwise prediction of conformational discontinuous B-cell epitopes using the Mapitope algorithm. Proteins 2007, 68:294–304.View ArticlePubMedGoogle Scholar
- Ponomarenko J, Bui H-H, Li W, Fusseder N, Bourne P, Sette A, Peters B: ElliPro: a new structure-based tool for the prediction of antibody epitopes. BMC Bioinformatics 2008, 9:514.View ArticlePubMedGoogle Scholar
- Li W, Godzik A: Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 2006, 22:1658–1659.View ArticlePubMedGoogle Scholar
- Garnier J, Gibrat JF, Robson B: GOR method for predicting protein secondary structure from amino acid sequence. Methods Enzymol 1996, 266:540–553.View ArticlePubMedGoogle Scholar
- Ansari HR, Raghava GP: Identification of NAD interacting residues in proteins. BMC Bioinformatics 2010, 11:160.View ArticlePubMedGoogle Scholar
- Kumar M, Gromiha MM, Raghava GP: Prediction of RNA binding sites in a protein using SVM and PSSM profile. Proteins 2008, 71:189–194.View ArticlePubMedGoogle Scholar
- Bhasin M, Raghava GP: Pcleavage: an SVM based method for prediction of constitutive proteasome and immunoproteasome cleavage sites in antigenic sequences. Nucleic Acids Res 2005, 33:W202–207.View ArticlePubMedGoogle Scholar
- Chou KC, Shen HB: Signal-CF: a subsite-coupled and window-fusing approach for predicting signal peptides. Biochem Biophys Res Commun 2007, 357:633–640.View ArticlePubMedGoogle Scholar
- Xiao X, Wang P, Chou KC: GPCR-CA: A cellular automaton image approach for predicting G-protein-coupled receptor functional classes. J Comput Chem 2009, 30:1414–1423.View ArticlePubMedGoogle Scholar
- Xiao X, Shao S, Ding Y, Huang Z, Chou KC: Using cellular automata images and pseudo amino acid composition to predict protein subcellular location. Amino Acids 2006, 30:49–54.View ArticlePubMedGoogle Scholar
- Rubinstein ND, Mayrose I, Martz E, Pupko T: Epitopia: a web-server for predicting B-cell epitopes. BMC Bioinformatics 2009, 10:287.View ArticlePubMedGoogle Scholar
- Grantham R: Amino acid difference formula to help explain protein evolution. Science 1974, 185:862–864.View ArticlePubMedGoogle Scholar
- Karplus PA, Schulz GE: Prediction of Chain Flexibility in Proteins - A tool for the Selection of Peptide Antigens. Naturwissenschafren 1985, 72:212–213.View ArticleGoogle Scholar
- Kolaskar AS, Tongaonkar PC: A semi-empirical method for prediction of antigenic determinants on protein antigens. FEBS Lett 1990, 276:172–174.View ArticlePubMedGoogle Scholar
- Parker JM, Guo D, Hodges RS: New hydrophilicity scale derived from high-performance liquid chromatography peptide retention data: correlation of predicted surface residues with antigenicity and X-ray-derived accessible sites. Biochemistry 1986, 25:5425–5432.View ArticlePubMedGoogle Scholar
- Ponnuswamy PK, Prabhakaran M, Manavalan P: Hydrophobic packing and spatial arrangement of amino acid residues in globular proteins. Biochim Biophys Acta 1980, 623:301–316.PubMedGoogle Scholar
- Kaundal R, Raghava GP: RSLpred: an integrative system for predicting subcellular localization of rice proteins combining compositional and evolutionary information. Proteomics 2009, 9:2324–2342.View ArticlePubMedGoogle Scholar
- Bhasin M, Raghava GP: GPCRpred: an SVM-based method for prediction of families and subfamilies of G-protein coupled receptors. Nucleic Acids Res 2004, 32:W383–389.View ArticlePubMedGoogle Scholar
- Chen C, Chen L, Zou X, Cai P: Prediction of protein secondary structure content by using the concept of Chou's pseudo amino acid composition and support vector machine. Protein Pept Lett 2009, 16:27–31.View ArticlePubMedGoogle Scholar
- Chen J, Liu H, Yang J, Chou KC: Prediction of linear B-cell epitopes using amino acid pair antigenicity scale. Amino Acids 2007, 33:423–428.View ArticlePubMedGoogle Scholar
- Yang ZR: Biological applications of support vector machines. Brief Bioinform 2004, 5:328–338.View ArticlePubMedGoogle Scholar
- Chou KC, Zhang CT: Prediction of protein structural classes. Crit Rev Biochem Mol Biol 1995, 30:275–349.View ArticlePubMedGoogle Scholar
- Chou KC, Shen HB: Cell-PLoc: a package of Web servers for predicting subcellular localization of proteins in various organisms. Nat Protoc 2008, 3:153–162.View ArticlePubMedGoogle Scholar
- Chou KC, Shen HB: A new method for predicting the subcellular localization of eukaryotic proteins with both single and multiple sites: Euk-mPLoc 2.0. PLoS ONE 2010, 5:e9931.View ArticlePubMedGoogle Scholar
- Vacic V, Iakoucheva LM, Radivojac P: Two Sample Logo: a graphical representation of the differences between two sets of sequence alignments. Bioinformatics 2006, 22:1536–1537.View ArticlePubMedGoogle Scholar
Copyright
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.