What is Gibbs Free Energy?
| Gibbs Free Energy is invented by Josiah Willard Gibbs (February 11, 1839 – April 28, 1903) – an American scientist who made important theoretical contributions to physics, chemistry, and mathematics. More on Josiah Willard Gibbs |
Gibbs free energy is used to predict whether a chemical process is spontaneous or non-spontaneous.
You might be wondering why we need to know that. Well, there are many applications where advance knowledge of whether a chemical reaction will proceed or not is important. For example in the manufacturing of products – if you are unable to predict which direction your process is going then you might be wasting resources producing unwanted products.
Spontaneous Process
A spontaneous process will proceed naturally. While you may have to provide some activation energy, the rest will proceed without the need of a continuous input of an external source of energy. A good example of a spontaneous process is combustion – the burning of substances to produce heat and energy. You may have to light the substance first (giving it activation energy) but once the substance is burning, it continues by itself.
Non-spontaneous Process
In a non-spontaneous process, a continuous energy input is necessary for the reaction to proceed.
A good example is photosynthesis, a process in which plants use to make glucose. Photosynthesis requires energy (in the form of sunlight) to drive chemical reactions. Therefore at night when it’s dark, photosynthesis does not work.
Spontaneous vs non-spontaneous process
| A spontaneous process – no continuous energy input from external source is necessary to run the process. | A non-spontaneous process – a continuous energy input is necessary to run the process. |
The Gibb’s Free Energy is simply a method of telling whether a chemical process will take place spontaneously or non-spontaneously.
- The Gibbs free energy calculates the amount of energy available in a system to do work. Eg. providing the activation energy for another chemical reaction.
- Gibbs free energy is only applicable for systems under constant temperature and pressure.
- The Gibbs calculation allows you to determine whether a process is spontaneous or not.
- The Gibbs calculation uses the relationship between enthalpy and entropy in a system to determine the spontaneity of a process.
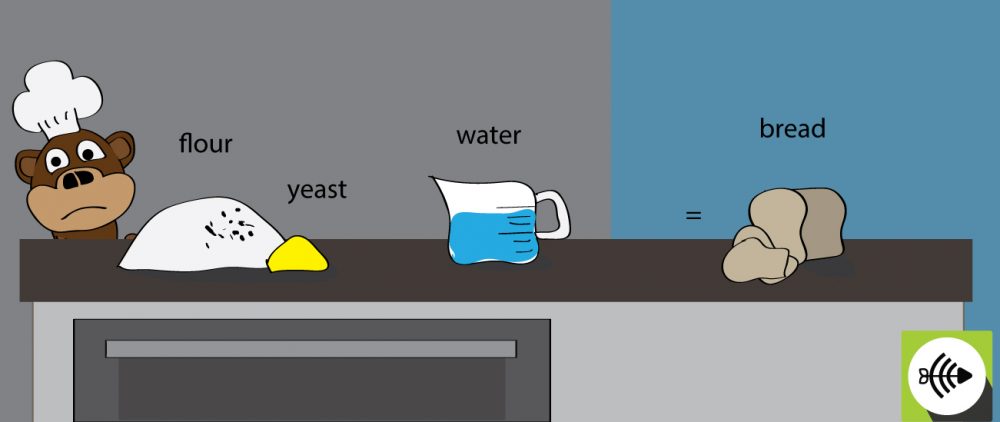
Is bread making a spontaneous or non-spontaneous reaction?
When yeast is added to the flour, the fermentation process is spontaneous. This occurs naturally. For the bread to cook, it needs to be baked in the oven at a high temperature. The baking process requires a continuous input of energy and therefore, the process is non-spontaneous.
Using Gibbs Free Energy
Gibbs Free Energy is the energy associated with a chemical reaction that can be used to do work.
∆G – Gibbs free energy
If Gibbs free energy for a reaction is negative, the reaction is spontaneous
Whether spontaneous or non-spontaneous
| Gibbs free energy | Less than 0 | Greater than 0 |
| ΔG | spontaneous | non-spontaneous |


Superb..it just easy to learn and understand
Very useful thanku
I love this site so much you go way Down simplifying things and making it easy for people to understand
Love this explanation
Very informative