What is Obesity ?
Overweight and Obesity are defined as “abnormal or excessive fat accumulation that may impair health” (the World Health Organization).
Obesity (BMI of ≥30 kg/m2) is defined as an excessively high amount of body fat or adipose tissue in relation to lean body mass. Obesity is caused by a chronic energy imbalance involving both dietary intake and physical activity patterns. Evidence shows that increased energy intake is causing the rise in obesity 1, 2, 3, 4, 5, 6 which is a result of changes in the global food system: the movement from individual to mass preparation “lowered the time price of food consumption” 2 and produced more highly processed food (with added sugar, fats, salt, and flavour enhancers), and marketed them with increasingly effective techniques. Additionally, marketing of food and beverages is associated with increasing obesity rates 7 and is especially effective among children 8, 9. Other factors such as wealth, government policy, cultural norms, the built environment 1, genetic 10 and epigenetic mechanisms 11, biological bases for food preferences 12, and biological mechanisms that regulate motivation for physical activity 13 all influence growth of the obesity epidemic.
To measure obesity, researchers commonly use a scale known as the body mass index (BMI). Body mass index (BMI) is calculated by dividing a person’s weight (in kilograms) by their height (in meters) squared (commonly expressed as kg/m2). BMI provides a more accurate measure of obesity than weight alone, and for most people it is a fairly good (although indirect) indicator of body fatness. Other measurements that reflect the distribution of body fat—that is, whether more fat is carried around the hips or the abdomen—are increasingly being used along with BMI as indicators of obesity and disease risks. These measurements include waist circumference and the waist-to-hip ratio (the waist circumference divided by the hip circumference). Overweight (BMI of 25 to 29.9 kg/m2) refers to increased body weight in relation to height, which is then compared to a standard of acceptable weight.
- BMI Calculator Adults. https://www.cdc.gov/healthyweight/assessing/bmi/adult_BMI/english_bmi_calculator/bmi_calculator.html
- BMI Calculator Children. https://nccd.cdc.gov/dnpabmi/Calculator.aspx
To find out about your body mass index (BMI), you can use a FREE online BMI calculators from the Centers for Disease Control and Prevention (CDC) – for
Compared with people of normal weight, those who are overweight or obese are at greater risk for many diseases, including type 2 diabetes, high blood pressure, cardiovascular disease, stroke, coronary heart disease and many cancers 16, 17, 18. Extreme or severe obesity is also associated with an increased death rate; heart disease, cancer, and diabetes are responsible for most of the excess deaths 19, 20.
A major cause of cancer is excessive obesity [adiposity] 21. This paradigm, also referred to as positive energy balance, is here to stay, because the evidence is overwhelming from all types of studies. These findings have coalesced from research over the last 10 to 15 years, but the evidence to support this idea actually goes back to animal studies in the 1930s. On a population level, the number of cases of cancer attributable to people being overweight and obese is about equal to the number attributable to current smoking. This is in part because smoking is going down and obesity is going up; in terms of importance within a population, they are in the same ballpark. However, on an individual basis, the cancer risk due to smoking remains substantially higher than that due to obesity. People who are obese have an increased risk of several types of cancer, including cancers of the breast (in women who have been through menopause), colon, rectum, endometrium (lining of the uterus), esophagus, kidney, pancreas, and gallbladder 22. A 2016 study summarizing worldwide estimates of the fractions of different cancers attributable to overweight/obesity reported that, compared with other countries, the United States had the highest fractions attributable to overweight/obesity for colorectal cancer, pancreatic cancer, and postmenopausal breast cancer 23. A population-based study using BMI and cancer incidence data from the GLOBOCAN project estimated that, in 2012 in the United States, about 28,000 new cases of cancer in men (3.5%) and 72,000 in women (9.5%) were due to overweight or obesity 24. The percentage of cases attributed to overweight or obesity varied widely for different cancer types but was as high as 54% for gallbladder cancer in women and 44% for esophageal adenocarcinoma in men. A 2016 study summarizing worldwide estimates of the fractions of different cancers attributable to overweight/obesity reported that, compared with other countries, the United States had the highest fractions attributable to overweight/obesity for colorectal cancer, pancreatic cancer, and postmenopausal breast cancer 23.
Compared with people of normal weight, those who are overweight or obese are at greater risk for many diseases, including diabetes, high blood pressure, cardiovascular disease, stroke, atherosclerosis, sleep disorders and many cancers 25. Extreme or severe obesity is also associated with an increased death rate; heart disease, cancer, and diabetes are responsible for most of the excess deaths 26, 27.
Conversely, eating a healthy diet, being physically active, and keeping a healthy weight may help reduce risk of some cancers. These healthy behaviors are also important to lessen the risk of other illnesses, such as heart disease, type II diabetes, and high blood pressure 22.
To measure obesity, researchers commonly use a scale known as the body mass index (BMI). BMI is calculated by dividing a person’s weight (in kilograms) by their height (in meters) squared (commonly expressed as kg/m2). The National Heart Lung and Blood Institute has a BMI calculator at 28. BMI provides a more accurate measure of obesity than weight alone, and for most people it is a fairly good (although indirect) indicator of body fatness.
Other measurements that reflect the distribution of body fat—that is, whether more fat is carried around the hips or the abdomen—are increasingly being used along with BMI as indicators of obesity and disease risks. These measurements include waist circumference and the waist-to-hip ratio (the waist circumference divided by the hip circumference).
The standard weight categories based on BMI for adults age 20 years or older are:
| BMI in kg/m2 | Weight Category |
|---|---|
| Below 18.5 | Underweight |
| 18.5 to 24.9 | Normal |
| 25.0 to 29.9 | Overweight |
| 30.0 to 39.9 | Obese |
| 40.0 or higher | Severely obese |
For children and adolescents (younger than 20 years of age), overweight and obesity are based on the Centers for Disease Control and Prevention’s (CDC’s) BMI-for-age growth charts, which are available at 29.
The CDC has a BMI percentile calculator for children and teens at 30.
What causes obesity
Energy imbalances
Energy imbalances can cause overweight and obesity. Energy imbalances cause your body to store fat. An energy imbalance means that your energy IN does not equal your energy OUT. This energy is measured in calories. Energy IN is the amount of calories you get from food and drinks. Energy OUT is the amount of calories that your body uses for things such as breathing, digesting, being physically active, and regulating body temperature.
Overweight and obesity develop over time when you take in more calories than you use, or when energy IN is more than your energy OUT. This type of energy imbalance causes your body to store fat.
Weight stability requires a balance between calories consumed (calories IN) and calories expended (calories Out). Managing calorie intake is fundamental to achieving and maintaining calorie balance—the balance between the calories intake from foods and the calories expended from metabolic processes and physical activity. The best way to determine whether your eating pattern is at an appropriate number of calories is to monitor your body weight and adjust your calorie intake and energy expenditure in physical activity based on changes in your body weight over time.
All foods and many beverages contain calories, and the total number of calories varies depending on the macronutrients in a food. On average, carbohydrates and protein contain 4 calories per gram (17 kJ/g), fats contain 9 calories per gram (37 kJ/g) and alcohol (ethanol) has 7 calories per gram (29 kJ/g) and organic acid 3 calories per gram (13 kJ/g). The total number of calories you need each day varies depending on a number of factors, including the your age, sex, height, weight, your build (muscular or athletic or average or overweight) and level of physical activity. In addition, a need to lose, maintain, or gain weight and other factors affect how many calories should be consuming.
Your body uses certain nutrients such as carbohydrates or sugars, proteins, and fats from the foods you eat to:
- make energy for immediate use to power routine daily body functions and physical activity.
- store energy for future use by your body. Sugars are stored asglycogen in the liver and muscles. Fats are stored mainly as triglyceride in fat tissue.
The amount of energy that your body gets from the food you eat depends on the type of foods you eat, how the food is prepared, and how long it has been since you last ate.
The body has three types of fat tissue—white, brown, and beige—that it uses to fuel itself, regulate its temperature in response to cold, and store energy for future use. Learn about the role of each fat type in maintaining energy balance in the body.
- White fat tissue can be found around the kidneys and under the skin in the buttocks, thighs, and abdomen. This fat type stores energy, makes hormone that control the way the body regulates urges to eat or stop eating, and makes inflammatory substances that can lead to complications.
- Brown fat tissue is located in the upper back area of human infants. This fat type releases stored energy as heat energy when a baby is cold. It also can make inflammatory substances. Brown fat can be seen in children and adults.
- Beige fat tissue is seen in the neck, shoulders, back, chest and abdomen of adults and resembles brown fat tissue. This fat type, which uses carbohydrates and fats to produce heat, increases when children and adults are exposed to cold.
Some genetic syndromes and endocrine disorders can cause overweight or obesity.
Genetic syndromes
Several genetic syndromes are associated with overweight and obesity, including the following.
- Prader-Willi syndrome: Prader-Willi syndrome is a complex genetic condition that affects many parts of the body. Prader-Willi syndrome affects an estimated 1 in 10,000 to 30,000 people worldwide. In infancy, this condition is characterized by weak muscle tone (hypotonia), feeding difficulties, poor growth, and delayed development. Beginning in childhood, affected individuals develop an insatiable appetite, which leads to chronic overeating (hyperphagia) and obesity. Some people with Prader-Willi syndrome, particularly those with obesity, also develop type 2 diabetes (the most common form of diabetes). People with Prader-Willi syndrome typically have mild to moderate intellectual impairment and learning disabilities. Behavioral problems are common, including temper outbursts, stubbornness, and compulsive behavior such as picking at the skin. Sleep abnormalities can also occur. Additional features of this condition include distinctive facial features such as a narrow forehead, almond-shaped eyes, and a triangular mouth; short stature; and small hands and feet. Some people with Prader-Willi syndrome have unusually fair skin and light-colored hair. Both affected males and affected females have underdeveloped genitals. Puberty is delayed or incomplete, and most affected individuals are unable to have children (infertile).Prader-Willi syndrome is caused by the loss of function of genes in a particular region of chromosome 15. People normally inherit one copy of this chromosome from each parent. Some genes are turned on (active) only on the copy that is inherited from a person’s father (the paternal copy). This parent-specific gene activation is caused by a phenomenon called genomic imprinting.Most cases of Prader-Willi syndrome (about 70 percent) occur when a segment of the paternal chromosome 15 is deleted in each cell. People with this chromosomal change are missing certain critical genes in this region because the genes on the paternal copy have been deleted, and the genes on the maternal copy are turned off (inactive). In another 25 percent of cases, a person with Prader-Willi syndrome has two copies of chromosome 15 inherited from his or her mother (maternal copies) instead of one copy from each parent. This phenomenon is called maternal uniparental disomy. Rarely, Prader-Willi syndrome can also be caused by a chromosomal rearrangement called a translocation, or by a mutation or other defect that abnormally turns off (inactivates) genes on the paternal chromosome 15.It appears likely that the characteristic features of Prader-Willi syndrome result from the loss of function of several genes on chromosome 15. Among these are genes that provide instructions for making molecules called small nucleolar RNAs (snoRNAs). These molecules have a variety of functions, including helping to regulate other types of RNA molecules. (RNA molecules play essential roles in producing proteins and in other cell activities.) Studies suggest that the loss of a particular group of snoRNA genes, known as the SNORD116 cluster, may play a major role in causing the signs and symptoms of Prader-Willi syndrome. However, it is unknown how a missing SNORD116 cluster could contribute to intellectual disability, behavioral problems, and the physical features of the disorder. In some people with Prader-Willi syndrome, the loss of a gene called OCA2 is associated with unusually fair skin and light-colored hair. The OCA2 gene is located on the segment of chromosome 15 that is often deleted in people with this disorder. However, loss of the OCA2 gene does not cause the other signs and symptoms of Prader-Willi syndrome. The protein produced from this gene helps determine the coloring (pigmentation) of the skin, hair, and eyes.Researchers are studying other genes on chromosome 15 that may also be related to the major signs and symptoms of Prader-Willi syndrome.
- Congenital leptin deficiency: Congenital leptin deficiency is a condition that causes severe obesity beginning in the first few months of life. Congenital leptin deficiency is a rare disorder. Only a few dozen cases have been reported in the medical literature. Without treatment, the extreme hunger continues and leads to chronic excessive eating (hyperphagia) and obesity. Beginning in early childhood, affected individuals develop abnormal eating behaviors such as fighting with other children over food, hoarding food, and eating in secret. People with congenital leptin deficiency also have hypogonadotropic hypogonadism, which is a condition caused by reduced production of hormones that direct sexual development. Without treatment, affected individuals experience delayed puberty or do not go through puberty, and may be unable to conceive children (infertile).Congenital leptin deficiency is caused by mutations in the LEP gene. This gene provides instructions for making a hormone called leptin, which is involved in the regulation of body weight. Normally, the body’s fat cells release leptin in proportion to their size. As fat accumulates in cells, more leptin is produced. This rise in leptin indicates that fat stores are increasing. Leptin attaches (binds) to and activates a protein called the leptin receptor, fitting into the receptor like a key into a lock. The leptin receptor protein is found on the surface of cells in many organs and tissues of the body including a part of the brain called the hypothalamus. The hypothalamus controls hunger and thirst as well as other functions such as sleep, moods, and body temperature. It also regulates the release of many hormones that have functions throughout the body. In the hypothalamus, the binding of leptin to its receptor triggers a series of chemical signals that affect hunger and help produce a feeling of fullness (satiety). LEP gene mutations that cause congenital leptin deficiency lead to an absence of leptin. As a result, the signaling that triggers feelings of satiety does not occur, leading to the excessive hunger and weight gain associated with this disorder. Because hypogonadotropic hypogonadism occurs in congenital leptin deficiency, researchers suggest that leptin signaling is also involved in regulating the hormones that control sexual development. However, the specifics of this involvement and how it may be altered in congenital leptin deficiency are unknown. Congenital leptin deficiency is a rare cause of obesity. Researchers are studying the factors involved in more common forms of obesity.
- Proopiomelanocortin deficiency: Proopiomelanocortin (POMC gene) deficiency causes severe obesity that begins at an early age. In addition to obesity, people with this condition have low levels of a hormone known as adrenocorticotropic hormone (ACTH) and tend to have red hair and pale skin. POMC deficiency is a rare condition; approximately 50 cases have been reported in the medical literature. Affected infants are usually a normal weight at birth, but they are constantly hungry, which leads to excessive feeding (hyperphagia). The babies continuously gain weight and are severely obese by age 1. Affected individuals experience excessive hunger and remain obese for life. It is unclear if these individuals are prone to weight-related conditions like cardiovascular disease or type 2 diabetes. Low levels of ACTH lead to a condition called adrenal insufficiency, which occurs when the pair of small glands on top of the kidneys (the adrenal glands) do not produce enough hormones. Adrenal insufficiency often results in periods of severely low blood sugar (hypoglycemia) in people with POMC deficiency, which can cause seizures, elevated levels of a toxic substance called bilirubin in the blood (hyperbilirubinemia), and a reduced ability to produce and release a digestive fluid called bile (cholestasis). Without early treatment, adrenal insufficiency can be fatal. Pale skin that easily burns when exposed to the sun and red hair are common in POMC deficiency, although not everyone with the condition has these characteristics. POMC deficiency is caused by mutations in the POMC gene, which provides instructions for making the proopiomelanocortin protein. This protein is cut (cleaved) into smaller pieces called peptides that have different functions in the body. One of these peptides, ACTH, stimulates the release of another hormone called cortisol from the adrenal glands. Cortisol is involved in the maintenance of blood sugar levels. Another peptide, alpha-melanocyte stimulating hormone (α-MSH), plays a role in the production of the pigment that gives skin and hair their color. The α-MSH peptide and another peptide called beta-melanocyte stimulating hormone (β-MSH) act in the brain to help maintain the balance between energy from food taken into the body and energy spent by the body. The correct balance is important to control eating and weight. POMC gene mutations that cause POMC deficiency result in production of an abnormally short version of the POMC protein or no protein at all. As a result, there is a shortage of the peptides made from POMC, including ACTH, α-MSH, and β-MSH. Without ACTH, there is a reduction in cortisol production, leading to adrenal insufficiency. Decreased α-MSH in the skin reduces pigment production, resulting in the red hair and pale skin often seen in people with POMC deficiency. Loss of α-MSH and β-MSH in the brain dysregulates the body’s energy balance, leading to overeating and severe obesity. POMC deficiency is a rare cause of obesity; POMC gene mutations are not frequently associated with more common, complex forms of obesity. Researchers are studying other factors that are likely involved in these forms.
- Bardet-Biedl syndrome: Bardet-Biedl syndrome is a disorder that affects many parts of the body. The signs and symptoms of this condition vary among affected individuals, even among members of the same family. Vision loss is one of the major features of Bardet-Biedl syndrome. Loss of vision occurs as the light-sensing tissue at the back of the eye (the retina) gradually deteriorates. Problems with night vision become apparent by mid-childhood, followed by blind spots that develop in the side (peripheral) vision. Over time, these blind spots enlarge and merge to produce tunnel vision. Most people with Bardet-Biedl syndrome also develop blurred central vision (poor visual acuity) and become legally blind by adolescence or early adulthood. Obesity is another characteristic feature of Bardet-Biedl syndrome. Abnormal weight gain typically begins in early childhood and continues to be an issue throughout life. Complications of obesity can include type 2 diabetes, high blood pressure (hypertension), and abnormally high cholesterol levels (hypercholesterolemia). Other major signs and symptoms of Bardet-Biedl syndrome include the presence of extra fingers or toes (polydactyly), intellectual disability or learning problems, and abnormalities of the genitalia. Most affected males produce reduced amounts of sex hormones (hypogonadism), and they are usually unable to father biological children (infertile). Many people with Bardet-Biedl syndrome also have kidney abnormalities, which can be serious or life-threatening. Additional features of Bardet-Biedl syndrome can include impaired speech, delayed development of motor skills such as standing and walking, behavioral problems such as emotional immaturity and inappropriate outbursts, and clumsiness or poor coordination. Distinctive facial features, dental abnormalities, unusually short or fused fingers or toes, and a partial or complete loss of the sense of smell (anosmia) have also been reported in some people with Bardet-Biedl syndrome. Additionally, this condition can affect the heart, liver, and digestive system. In most of North America and Europe, Bardet-Biedl syndrome has a prevalence of 1 in 140,000 to 1 in 160,000 newborns. The condition is more common on the island of Newfoundland (off the east coast of Canada), where it affects an estimated 1 in 17,000 newborns. It also occurs more frequently in the Bedouin population of Kuwait, affecting about 1 in 13,500 newborns. Bardet-Biedl syndrome can result from mutations in at least 14 different genes (often called BBS genes). These genes are known or suspected to play critical roles in cell structures called cilia. Cilia are microscopic, finger-like projections that stick out from the surface of many types of cells. They are involved in cell movement and many different chemical signaling pathways. Cilia are also necessary for the perception of sensory input (such as sight, hearing, and smell). The proteins produced from BBS genes are involved in the maintenance and function of cilia.
- Alström syndrome: Alström syndrome is a rare condition that affects many body systems. Many of the signs and symptoms of this condition begin in infancy or early childhood, although some appear later in life. Alström syndrome is characterized by a progressive loss of vision and hearing, a form of heart disease that enlarges and weakens the heart muscle (dilated cardiomyopathy), obesity, type 2 diabetes (the most common form of diabetes), and short stature. This disorder can also cause serious or life-threatening medical problems involving the liver, kidneys, bladder, and lungs. Some individuals with Alström syndrome have a skin condition called acanthosis nigricans, which causes the skin in body folds and creases to become thick, dark, and velvety. The signs and symptoms of Alström syndrome vary in severity, and not all affected individuals have all of the characteristic features of the disorder. More than 900 people with Alström syndrome have been reported worldwide. Mutations in the ALMS1 gene cause Alström syndrome. The ALMS1 gene provides instructions for making a protein whose function is unknown. Mutations in this gene probably lead to the production of an abnormally short, nonfunctional version of the ALMS1 protein. This protein is normally present at low levels in most tissues, so a loss of the protein’s normal function may help explain why the signs and symptoms of Alström syndrome affect many parts of the body.
- Cohen syndrome: Cohen syndrome is an inherited disorder that affects many parts of the body and is characterized by developmental delay, intellectual disability, small head size (microcephaly), and weak muscle tone (hypotonia). Other features common in this condition include worsening nearsightedness (myopia), breakdown (degeneration) of the light-sensitive tissue at the back of the eye (retinal dystrophy), an unusually large range of joint movement (hypermobility), and distinctive facial features. These facial features typically include thick hair and eyebrows, long eyelashes, unusually-shaped eyes (down-slanting and wave-shaped), a bulbous nasal tip, a smooth or shortened area between the nose and the upper lip (philtrum), and prominent upper central teeth. The combination of the last two facial features results in an open mouth. The features of Cohen syndrome vary widely among affected individuals. Additional signs and symptoms in some individuals with this disorder include low levels of white blood cells (neutropenia), overly friendly behavior, and obesity that develops in late childhood or adolescence. When obesity is present, it typically occurs around the torso, with the arms and legs remaining slender (called truncal obesity). Individuals with Cohen syndrome may also have narrow hands and feet, and slender fingers. The exact incidence of Cohen syndrome is unknown. It has been diagnosed in fewer than 1,000 people worldwide. More cases are likely undiagnosed. Mutations in the VPS13B gene (also called the COH1 gene) cause Cohen syndrome. The protein produced from this gene is a part of the Golgi apparatus, which is a cell structure in which newly produced proteins are modified so they can carry out their functions. In particular, the VPS13B protein is involved in a modification called glycosylation, which is the attachment of sugar molecules to proteins. The VPS13B protein also appears to be involved in the sorting and transporting of proteins inside the cell. This protein is thought to be involved in normal growth and development of nerve cells (neurons) and fat cells (adipocytes), and may play a role in the storage and distribution of fats in the body.Most mutations in the VPS13B gene are believed to prevent the production of functional VPS13B protein. Studies suggest that a loss of this protein disrupts the organization of the Golgi apparatus and impairs normal glycosylation. However, it is not known how a lack of functional VPS13B protein or these cellular changes lead to the signs and symptoms of Cohen syndrome. Researchers speculate that problems with neuron development underlie microcephaly, intellectual disability, and retinal dystrophy and that abnormal fat storage may cause truncal obesity in people with Cohen syndrome.
The study of these genetic syndromes has helped researchers understand obesity.
Endocrine disorders
Because the endocrine system produces hormones that help maintain energy balances in the body, the following endocrine disorders or tumors affecting the endocrine system can cause overweight and obesity.
- Hypothyroidism (underactive thyroid gland). People with this condition have low levels of thyroid hormones. These low levels are associated with decreased metabolism and weight gain, even when food intake is reduced. People with hypothyroidism also produce less body heat, have a lower body temperature, and do not efficiently use stored fat for energy.
- Cushing’s syndrome. People with this condition have high levels of glucocorticoids, such as cortisol, in the blood. High cortisol levels make the body feel like it is underchronic stress. As a result, people have an increase in appetite and the body will store more fat. Cushing’s syndrome may develop after taking certain medicines or because the body naturally makes too much cortisol.
- Tumors. Some tumors, such as craneopharingioma, can cause severe obesity because the tumors develop near parts of the brain that control hunger.
Stress Causes People to Overeat
There is much truth behind the phrase “stress eating” 31. Indeed, individuals attempting to lose weight often cite life stressors as reasons for abandoning diet plans, and following strict diet/exercise programs is a stressful endeavour. Rigidly restricting food intake often leads to compensatory overeating and psychological distress associated with diet-breaking may further increase disinhibited eating 32. Researchers have linked weight gain to stress 33 and according to an American Psychological Association survey, about one-fourth of Americans rate their stress level as 8 or more on a 10-point scale 34.
In the short term, stress can shut down appetite. A structure in the brain called the hypothalamus produces corticotropin-releasing hormone, which suppresses appetite 35. The brain also sends messages to the adrenal glands atop the kidneys to pump out the hormone epinephrine (also known as adrenaline). Epinephrine helps trigger the body’s fight-or-flight response, a revved-up physiological state that temporarily puts eating on hold.
But if stress persists, it’s a different story. The adrenal glands release another hormone called cortisol, and cortisol increases appetite and may also ramp up motivation in general, including the motivation to eat 36. Once a stressful episode is over, cortisol levels should fall, but if the stress doesn’t go away — or if a person’s stress response gets stuck in the “on” position — cortisol may stay elevated 37.
Stress also seems to affect food preferences. Numerous studies, granted, many of them in animals — have shown that physical or emotional distress increases the intake of food high in fat, sugar, or both. High cortisol levels, in combination with high insulin levels, may be responsible. Other research suggests that ghrelin, a “hunger hormone,” may have a role 38, 39, 40. Stress-induced increases in ghrelin, which further stimulate appetite, might impede efforts to maintain weight loss. Interventions to prevent increased ghrelin levels in response to stress and weight loss are needed 41 .
Once ingested, fat- and sugar-filled foods seem to have a feedback effect that inhibits activity in the parts of the brain that produce and process stress and related emotions. These foods really are “comfort” foods in that they seem to counteract stress — and this may contribute to people’s stress-induced craving for those foods. Of course, overeating isn’t the only stress-related behavior that can add pounds. Stressed people also lose sleep (sleep deprivation), exercise less, and drink more alcohol, all of which can contribute to excess weight.
- Stress Gender differences
Some research suggests a gender difference in stress-coping behavior, with women being more likely to turn to food and men to alcohol or smoking. And a Finnish study that included over 5,000 men and women showed that obesity was associated with stress-related eating in women but not in men.
Harvard researchers 35 have reported that stress from work and other sorts of problems correlates with weight gain, but only in those who were overweight at the beginning of the study period. One theory is that overweight people have elevated insulin levels, and stress-related weight gain is more likely to occur in the presence of high insulin.
How much cortisol people produce in response to stress may also factor into the stress–weight gain equation. In 2007, British researchers designed an ingenious study that showed that people who responded to stress with high cortisol levels in an experimental setting were more likely to snack in response to daily hassles in their regular lives than low-cortisol responders.
- Steps to counter stress over eating
When stress affects someone’s appetite and waistline, the individual can forestall further weight gain by ridding the refrigerator and cupboards of high-fat, sugary foods. Keeping those “comfort foods” handy is just inviting trouble.
Here are some other suggestions for countering stress:
- Meditation. Countless studies show that meditation reduces stress, although much of the research has focused on high blood pressure and heart disease. Meditation may also help people become more mindful of food choices. With practice, a person may be able to pay better attention to the impulse to grab a fat- and sugar-loaded comfort food and inhibit the impulse 42 .
- Exercise. Intense exercise increases cortisol levels temporarily, but low-intensity exercise seems to reduce them. University of California researchers reported that exercise — and this was vigorous exercise — may blunt some of the negative effects of stress. Some activities, such as yoga and tai chi, have elements of both exercise and meditation.
- Social support. Friends, family, and other sources of social support seem to have a buffering effect on the stress that people experience. For example, research suggests that people working in stressful situations, like hospital emergency departments, have better mental health if they have adequate social support. But even people who live and work in situations where the stakes aren’t as high need help from time to time from friends and family.
IT IS AMAZINGLY EMPOWERING TO HAVE THE SUPPORT OF A STRONG, MOTIVATED AND INSPIRATIONAL GROUP OF PEOPLE.
The kind of people that makes you feel wonderful about yourself. They will give and offer support to your learning and growing. So that when you say you want to lose the excess body fat, or go back to school, or get a new job, or whatever, your inspirational and positive friends will say, “I think that is a fantastic idea. You’ll do beautifully. Don’t worry . . . you have what it takes! Go for it!”
Among the new friends you make, include those who are farther along the journey than you are at the moment. If you are to find our way across troubled waters, you are better served by the company of those who have built bridges, who have moved beyond despair and inertia.
Life becomes more fun and less of a struggle when you don’t have to pioneer on your own. There is a lightness about positive people. They have learned not to take themselves so seriously and they are a joy to be around.
We can’t stress enough how important it is to begin now to have strong people in your life, in the form of an established group or simply a group of friends who are consciously in the process of growing. It is incredibly important to your peace of mind and sense of power to have some kind of support group.
When you are concerned with something bigger than yourself, your fears are greatly diminished. You sense yourself as being part of a bigger whole—you are not alone and you, perhaps for the first time, are aware of a sense of purpose.
How Does Sleep Affect Your Body Weight
Researchers speculate that there are several ways that chronic sleep deprivation might lead to weight gain, either by increasing how much food people eat or decreasing the energy that they burn 43.
Sleep plays a vital role in good health and well-being throughout your life. Getting enough quality sleep at the right times can help protect your mental health, physical health, quality of life, and safety 44.
The way you feel while you’re awake depends in part on what happens while you’re sleeping 44. During sleep, your body is working to support healthy brain function and maintain your physical health. In children and teens, sleep also helps support growth and development.
The damage from sleep deficiency can occur in an instant (such as a car crash), or it can harm you over time. For example, ongoing sleep deficiency can raise your risk for some chronic health problems. It also can affect how well you think, react, work, learn, and get along with others.
There has been considerable debate in the scientific community about the importance of sleep, but some proposed functions of sleep are widely accepted:
- Restoration, providing time for the body to repair itself;
- Consolidation of memories;
- Enhancement of immune system function; and
- Maturation of the brain.
And the quality and amount of sleep you get can affect your mood, energy level, concentration and overall functioning. If you have sleep troubles, make sure that you have a quiet, relaxing bedtime routine, listen to soothing music, put clocks away, and stick to a consistent schedule.
The table below shows general recommendations for different age groups. This table reflects recent American Academy of Sleep Medicine recommendations that the American Academy of Pediatrics has endorsed.
Table 1. Recommended Amount of Sleep
| Age | Recommended Amount of Sleep |
|---|---|
| Infants aged 4-12 months | 12-16 hours a day (including naps) |
| Children aged 1-2 years | 11-14 hours a day (including naps) |
| Children aged 3-5 years | 10-13 hours a day (including naps) |
| Children aged 6-12 years | 9-12 hours a day |
| Teens aged 13-18 years | 8-10 hours a day |
| Adults aged 18 years or older | 7–8 hours a day |
Sleep deprivation could increase your energy intake by
Increasing hunger: Sleep deprivation may alter the hormones that control hunger 45. One small study, for example, found that young men who were deprived of sleep had higher levels of the appetite-stimulating hormone ghrelin and lower levels of the satiety-inducing hormone leptin, with a corresponding increase in hunger and appetite-especially for foods rich in fat and carbohydrates 46.
Giving you more time to eat: People who sleep less each night may eat more than people who get a full night’s sleep simply because they have more waking time available 47. Recently, a small laboratory study found that people who were deprived of sleep and surrounded by tasty snacks tended to snack more-especially during the extra hours they were awake at night-than when they had adequate sleep 48.
Prompting you to choose less healthy diets: Observational studies have not seen a consistent link between sleep and food choices 43. But one study of Japanese workers did find that workers who slept fewer than six hours a night were more likely to eat out, have irregular meal patterns, and snack than those who slept more than six hours 49.
Sleep deprivation could decrease your energy expenditure by
Decreasing your physical activity: People who don’t get enough sleep are more tired during the day, and as a result may curb their physical activity 50. Some studies have found that sleep-deprived people tend to spend more time watching TV, less time playing organized sports, and less time being physically active than people who get enough sleep. But these differences in physical activity or TV viewing are not large enough to explain the association between sleep and weight 51.
Lowering your body temperature: In laboratory experiments, people who are sleep-deprived tend to see a drop in their body temperatures 51. This drop, in turn, may lead to decreased energy expenditure. Yet a recent study did not find any link between sleep duration and total energy expenditure 52.
Sleep is a Promising Target for Obesity Prevention
There is convincing evidence that getting a less than ideal amount of sleep is an independent and strong risk factor for obesity, in infants and children as well as in adults. Most of the research thus far, however, has consisted of observational studies, and it remains to be seen whether teaching children or adults how to get a better night’s sleep can lower their risk of obesity or help them lose weight. Randomized clinical trials that are currently underway may soon provide more answers.
Some researchers have cautioned against being too quick to promote sleep as an answer to the obesity epidemic, given the shortcomings of the research conducted to date. Yet from a public health perspective, there is little risk in encouraging healthy sleep through lifestyle changes, such as setting a consistent bedtime, limiting caffeine late in the day, and curtailing high-tech distractions in the bedroom 53. Good sleep habits have other benefits, too, like boosting alertness at school or work, improving mood, and enhancing overall quality of life. That’s all the more reason to put a long night’s sleep on the short list for obesity prevention.
How common is overweight or obesity ?
Obesity is the result of people responding normally to the obesogenic environments they find themselves in. More than 78 million adults in the United States were obese in 2009–2010 54, 55. Current estimates, results from the National Health and Nutrition Examination Survey (NHANES) showed that in 2011–2014, nearly 70% of U.S. adults age 20 years or older were overweight or obese (overweight is defined as a BMI of 25 to 29.9 kg/m2 and obesity as a BMI of ≥30 kg/m2), and more than one-third (36.5%) were obese 56. In 1988–1994, by contrast, only 56% of adults aged 20 years or older were overweight or obese. If US trends based on historical data continue, the prevalence of obesity in US adults will increase from its present level of about 36.5% to about 50% by 2030, with increased costs of up to US$66 billion per year for treatment of obesity-associated diseases.
The percentage of children and adolescents who are overweight or obese has also increased 56. In 2011–2014, an estimated 9% of 2- to 5-year-olds, 17% of 6- to 11-year-olds, and 20% of 12- to 19-year-olds were overweight or obese. In 1988–1994, those figures were only 7%, 11%, and 10%, respectively. In 2011–2014, about 17% of U.S. youth ages 2 to 19 years old were obese. In 1988–1994, by contrast, only about 10% of 2 to 19-year old were obese 57.
Perhaps of greatest concern is the shift in the obese BMI distribution to a higher prevalence of ‘extreme obesity’ with BMI ≥40 kg/m2, which was 6.6 percent in years 2009–2010 58 and the latest available rates 54 indicate no decline in obesity rates. These latest data from the National Health and Nutrition Examination Survey (NHANES) report that for both men and women obesity estimates for 2009–2010 did not differ significantly from estimates for 2003–2008. Yet, overweight and obesity continue to be highly prevalent. Obesity disproportionately affects people from certain racial and ethnic groups and those who are socioeconomically disadvantaged.
According to the Centers for Disease Control and Prevention (CDC), the prevalence of obesity in the United States differs among racial/ethnic groups. For example, in 2011–2012 among adults, non-Hispanic blacks had the highest prevalence of obesity (47.8%) followed by Hispanics (42.0%), non-Hispanic whites (33.4%), and non-Hispanic Asians (10.9%) (5). Among children and adolescents ages 2–19 years, the prevalence of obesity in 2011–2012 was 21.9% among Hispanics, 19.5% among non-Hispanic blacks, 14.7% among non-Hispanic whites, and 8.6% among non-Hispanic Asians 59.
Trends in Overweight, Obesity, and Extreme Obesity Among Adults Aged 20 to 74 years: United States, 1960–1962 Through 2009–2010
(Source 60).
Note: Age-adjusted by the direct method to the year 2000 U.S. Bureau of the Census using age groups 20–39, 40–59 and 60–74 years. Pregnant females were excluded. Overweight defined as a BMI of 25 or greater but less than 30; obesity is a BMI greater than or equal to 30; extreme obesity is a BMI greater than or equal to 40.
- In Women, overweight and obesity are highest among non-Hispanic Black women (about 82 percent), compared with about 76 percent for Hispanic women and 64 percent for non-Hispanic White women.
- In Men, overweight and obesity are highest among Hispanic men (about 82 percent), compared with about 74 percent for non-Hispanic White men and about 70 percent for non-Hispanic Black men.
- In Children and Teens. Children also have become heavier. In the past 30 years, obesity has tripled among school-aged children and teens. According to the National Health and Nutrition Examination Survey 2009–2010, about 1 in 6 American children ages 2–19 are obese. The survey also suggests that overweight and obesity are having a greater effect on minority groups, including Blacks and Hispanics.
Approximately 17 percent of children and teens ages 2 through 19 also have obesity, and thus may be at increased risk for developing serious diseases both during their youth and later in adulthood 61, 62.
Overweight and obesity are linked to more deaths worldwide than underweight. Globally there are more people who are obese than underweight. Once considered a high-income country problem, overweight and obesity are now on the rise in low- and middle-income countries, particularly in urban settings.
As obesity has risen in the United States and all around the world, so too have many other obesity-related health conditions: diabetes, heart disease, stroke, cancer, and maybe even Alzheimer’s disease.
A research conducted by the Imperial College London highlighted the fact that obesity and associated diseases are the end result of a complex interplay between our genes, diets, lifestyles, and microbiomes.
The Brain’s Regulation of Appetite and Digestion
There is a seductive simplicity to the conceptualisation of obesity as a straightforward problem of energy balance—Calories IN versus Calories Out. But the physiological, behavioral, and environmental influences on this relation are asymmetrical. Although the basic arithmetic holds true, in practice it is much easier for people, and populations, to gain weight than to lose it. Increasing fatness is the result of a normal response, by normal people, to an abnormal situation. This holds true across the globe: although obesity is always thought of as a problem of the developed world, it is increasingly seen in developing nations too 63. Supporting and encouraging people to respond more healthily to that abnormal situation is important, but the range of options within which people make their choices is skewed in favor of weight gain rather than weight loss. No approach will work alone, but changing the environments within which those decisions are made is likely to be far more effective than merely exhorting people to make better choices 64.
New Insights into How the Brain Handles Hunger 65:
- Researchers have discovered new details about how the brain controls food intake in mice, suggesting a possible therapeutic target for curbing appetite.
In the central nervous system, hormones called melanocortins can activate signaling through the melanocortin 4 receptor (MC4R), affecting metabolism, food intake, and calorie burning (energy expenditure), as well as other physiological factors such as blood pressure. Genetic changes that inactivate the gene for MC4R can cause severe obesity. Without MC4R, food intake and body fat increase, while energy expenditure decreases and the body becomes less able to respond to the hormone insulin. Unfortunately, past attempts to activate MC4R’s effects as a potential obesity treatment also resulted in higher blood pressure, limiting its use as a therapy.
- A team of scientists 66 thus sought new insights into MC4R and proteins it partners with, as possible avenues to new treatment strategies. MC4R was known to work through a signaling protein called Gsα to affect energy expenditure and glucose metabolism in the brain. However, Gsα did not appear to be responsible for MC4R’s effects on reducing food intake, which originate in a different part of the brain, called the paraventricular nucleus (PVN). Thus, scientists hypothesized that another MC4R-triggered pathway was involved in food intake. To determine what this pathway might be, researchers investigated MC4R’s interactions with a pair of other signaling proteins, which they referred to collectively as Gq/11α. To determine if Gq/11α plays a role in regulating food intake, the researchers created genetically engineered mice lacking Gq/11α in the PVN and looked at the mice’s behavior, weight, and metabolism. Mice lacking Gq/11α ate more food and later, developed severe obesity compared to mice that still produced Gq/11α. This increased weight was
especially prominent in female mice. Additionally, a chemical that usually reduces food intake when injected into the PVN had a reduced effect in mice lacking Gq/11α in the PVN, demonstrating that Gq/11α was involved in curbing appetite. The mice lacking Gq/11α also developed elevated cholesterol levels. However, the lack of Gq/11α in the PVN had no effect on heart rate and blood pressure. These results confirmed that melanocortin’s effects on food intake and cholesterol levels in the PVN were mediated through Gq/11α, a different pathway than that which mediates melanocortin’s effects on blood pressure. - Overall, these results provide new clarity to the question of how hunger and obesity are regulated by the brain. They also offer new avenues for possible therapeutic interventions, since therapies that are specific to the Gq/11α pathway may be able to suppress appetite without unwanted cardiovascular side effects 66.
The Inner Workings of a Brain Cell That Drives Eating and Weight Gain:
- Seeking insights that could lead to novel obesity treatments, scientists discovered a molecular pathway in mouse brain cells that begins with activation of cell-surface proteins, called G protein-coupled receptors (GPCRs), and ends with the cells’ release of a powerful appetite-inducing molecule, AgRP. In this and previous research on AgRP neurons, the scientists also investigated another type of GPCR, and found that it, too, prompted mice to eat, but through a different molecular pathway. With multiple ways to provoke eating, the inner workings of these cells may pose a challenge to well-intentioned dieters, but they also present an opportunity. If future research reveals similar findings in people, scientists could develop drugs that interfere with steps along these brain cell pathways, with the goal of suppressing appetite to help individuals lose excess weight 67.
A Nutrient Sensor in Brain Cells Regulates Feeding:
- New research in mice has revealed a key enzyme that acts as a control switch for feeding. In this study, researchers investigated the function of the enzyme O-GlcNAc transferase (OGT) in male mice by knocking out the gene that encodes OGT (“OGT knockouts”) within a very specific set of neurons in the brains of adult mice. They then compared food intake and weight in mice without OGT to normal mice. They found that brain-specific loss of OGT caused a rapid weight gain in mice. Within 3 weeks, the genetically modified mice tripled their body fat. Although these mice ate only as frequently as their unmodified counterparts, they ate more at each meal. The researchers observed that if they restricted food access, the knockout mice maintained a normal weight. However, when free access to food was reintroduced, the mice quickly became obese 68.
How the Brain Knows When the Stomach Has Stretched and There Is Food To Digest:
- Exploring a biological data cable that transmits information from the gut to the brain, researchers discovered nerve cells in mice that detect nutrients to be digested, and other nerve cells that sense when the stomach and intestines have stretched in size to accommodate food just eaten. To regulate digestion and other processes, many nerve cells (neurons), bundled together to form the vagus nerve, monitor organs throughout the body and report back to the brain. Long fibers from this group of neurons permeate the stomach, intestines, or other organs at one end of the vagus nerve, while fibers at the other end reach up to connect to the brain. They found that GLP1R neurons projected their fibers into stomach and intestinal muscle, and that these cells became activated in response to stomach and intestinal stretching. By contrast, GPR65 neurons spread their fibers through intestinal structures that absorb nutrients and respond to food in the intestine. Thus, GLP1R and GPR65 neurons monitor and transmit different signals related to digestion. In other experiments, the researchers found that both types of neurons, when activated, could affect gut motility—the contractions and pressure that help digestion 69.
How What You Eat Can Affect How Much You Eat
The simultaneous increases in obesity in almost all countries seem to be driven mainly by changes in the global food system, which is producing more processed, affordable, and effectively marketed food than ever before. This passive over-consumption of energy leading to obesity is a predictable outcome of market economies predicated on consumption-based growth.
Scientists 70 identified one way the gut microbiome influences obesity and metabolism. A link between the bacteria that populate the intestines (part of the gut microbiome) and obesity had been previously discovered, but the details of how the microbiome influenced body weight were not known. To delve into this question, researchers built on a previous observation: changes in the amount of short-chain fatty acids (by-products of digestion in the gut) can be associated with overfeeding, obesity, and metabolic syndrome (factors that increase risk of heart disease and diabetes). In this new study, the scientists found that male rats fed a high-fat diet showed a striking increase in the amount of acetate, a short-chain fatty acid, in their bodies, and became insulin resistant, a condition associated with metabolic syndrome. Determining the origin and consequences of the increase in acetate resulted in an exciting discovery of how the gut microbiome affects metabolism.
By measuring the acetate in tissues of the rat, the scientists found the highest amount in the gut; treating the rats with antibiotics to kill the gut bacteria, or removing the colon (part of the gut), reduced the amount of acetate dramatically. Consistent with previous research, they also found that rats fed a high-fat diet had a mix of bacteria in their microbiome that was somewhat different from the gut bacteria of rats fed a normal diet. A fecal transfer—transplanting the gut microbiome from rats eating the high-fat diet into rats on a normal diet—also transferred the increase in acetate production. Together, these observations indicate that the gut microbiome was responsible for generating the increased acetate. To determine the chronic effects of increased acetate, rats on a normal diet received acetate infusions for 10 days. After this period, the rats had increased insulin secretion by the pancreatic β (beta) cells in response to insulin, were insulin resistant, and more than doubled their daily caloric intake and weight gain. Interestingly, the researchers discovered that the acetate stimulated the parasympathetic nervous system through the brain. These results suggest a model: exposure to a diet high in calories leads to increased acetate production by bacteria in the gut. The acetate enters the blood and travels to the brain. As a result, the brain signals to the pancreas to increase insulin secretion and storage of fat, and signals to the stomach to release the hunger hormone ghrelin. This process appears to lead to overfeeding and insulin resistance, creating a feedback loop. Additional research will be necessary to determine whether the same mechanism operates in humans and to identify which bacteria in the gut microbiome contribute to the production of acetate. Nevertheless this study describes a novel link between the gut microbiome, obesity, and metabolic syndrome that could be targeted in the development of therapeutics for obesity and diabetes 70.
Recent data indicate that the gut microbiome can predict body composition (lean or obese) with 90 percent accuracy compared to 60 percent accuracy with genetics alone. Moreover, evidence suggests the microbiome plays a critical role in weight loss interventions, but the precise functional nature of this role has not been clearly established 65.
Obesity Signs and Symptoms
Weight gain usually happens over time. Most people know when they’ve gained weight. Some of the signs of overweight or obesity include:
- Clothes feeling tight and needing a larger size.
- The scale showing that you’ve gained weight.
- Having extra fat around the waist.
- A higher than normal body mass index and waist circumference.
The body has three types of fat (adipose) tissue—white, brown, and beige—that it uses to fuel itself, regulate its temperature in response to cold, and store energy for future use.
- White fat tissue can be found around the kidneys and under the skin in the buttocks, thighs, and abdomen. This fat type stores energy, makes hormones that control the way the body regulates urges to eat or stop eating, and makes inflammatory substances that can lead to complications.
- Brown fat tissue is located in the upper back area of human infants. This fat type releases stored energy as heat energy when a baby is cold. It also can make inflammatory substances. Brown fat can be seen in children and adults.
- Beige fat tissue is seen in the neck, shoulders, back, chest and abdomen of adults and resembles brown fat tissue. This fat type, which uses carbohydrates and fats to produce heat, increases when children and adults are exposed to cold.
Several parts of your body, such as your stomach, intestines, pancreas, and fat tissue, use hormones to control how your brain decides if you are hungry or full. Some of these hormones are insulin, leptin, glucagon-like peptide (GLP-1), peptide YY, and ghrelin.
Adipose Tissue as an Endocrine Organ
As early as 1987, adipose tissue was identified as a major site for metabolism of sex steroids 71 and production of adipsin, an endocrine factor that is markedly down-regulated in rodent obesity 72. The subsequent identification and characterization of leptin in 1994 firmly established adipose tissue as an endocrine organ 73. Adipose tissue is now known to express and secrete a variety of bioactive peptides, known as adipokines, which act at both the local (autocrine/paracrine) and systemic (endocrine) level (Table 2). In addition to these efferent signals, adipose tissue expresses numerous receptors that allow it to respond to afferent signals from traditional hormone systems as well as the central nervous system (CNS) (Table 3). Thus, besides the biological repertoire necessary for storing and releasing energy, adipose tissue contains the metabolic machinery to permit communication with distant organs including the CNS. Through this interactive network, adipose tissue is integrally involved in coordinating a variety of biological processes including energy metabolism, neuroendocrine function, and immune function.
Table 2. Examples of adipocyte-derived proteins (adipokines) with endocrine functions
| Cytokines and cytokine-related proteins | Leptin |
| TNFα | |
| IL-6 | |
| Other immune-related proteins | MCP-1 |
| Proteins involved in the fibrinolytic system | PAI-1 |
| Tissue factor | |
| Complement and complement-related proteins | Adipsin (complement factor D) |
| Complement factor B | |
| ASP | |
| Adiponectin | |
| Lipids and proteins for lipid metabolism or transport | Lipoprotein lipase (LPL) |
| Cholesterol ester transfer protein (CETP) | |
| Apolipoprotein E | |
| NEFAs | |
| Enzymes involved in steroid metabolism | Cytochrome P450-dependent aromatase |
| 17βHSD | |
| 11βHSD1 | |
| Proteins of the RAS | AGT |
| Other proteins | Resistin |
Table 3. Examples of receptors expressed in adipose tissue that allow it to respond to afferent signals from traditional hormone systems as well as the central nervous system
| Receptors for traditional endocrine hormones | Insulin receptor |
| Glucagon receptor | |
| GH receptor | |
| TSH receptor | |
| Gastrin/CCK-B receptor | |
| Glucagon like peptide-1 receptor | |
| Angiotensin II receptors type 1 and 2 | |
| Nuclear hormone receptors | Glucocorticoid receptor |
| Vitamin D receptor | |
| Thyroid hormone receptor | |
| Androgen receptor | |
| Estrogen receptor | |
| Progesterone receptor | |
| Cytokine receptors | Leptin receptor |
| IL-6 receptor | |
| TNFα receptor | |
| Catecholamine receptors | β1, β2, β3 receptors |
| α1, α2 receptors |
The important endocrine function of adipose tissue is emphasized by the adverse metabolic consequences of both adipose tissue excess and deficiency. Adipose tissue excess or obesity, particularly in the visceral compartment, is associated with insulin resistance, hyperglycemia, dyslipidemia, hypertension, and prothrombotic and proinflammatory states 75. The prevalence of obesity and these associated morbidities, known as the metabolic syndrome, has reached epidemic proportions 75. Interestingly, adipose tissue deficiency or lipodystrophy is also associated with features of the metabolic syndrome in both humans and rodents 76. Furthermore, the prevalence of lipodystrophy in humans is increasing with the use of highly active antiretroviral therapy for HIV 76. Thus, both excess and deficiency of adipose tissue have harmful metabolic consequences and represent significant medical and socioeconomic burdens in the world today.
It is now clear that adipose tissue is a complex and highly active metabolic and endocrine organ 77, 78. Besides adipocytes, adipose tissue contains connective tissue matrix, nerve tissue, stromovascular cells, and immune cells 79. Although adipocytes express and secrete several endocrine hormones such as leptin and adiponectin, many secreted proteins are derived from the nonadipocyte fraction of adipose tissue 80. Regardless, these components function as an integrated unit, making adipose tissue a true endocrine organ 79. Here we present an overview of the endocrine functions of adipose tissue. These functions fall into two broad categories: 1) secreted proteins that have metabolic effects on distant cells or tissues, and 2) enzymes involved in the metabolism of steroid hormones.
Leptin
The effects of leptin on energy homeostasis are well documented 81. Many of these effects, particularly on energy intake and expenditure, are mediated via hypothalamic pathways, whereas other effects are mediated via direct action on peripheral tissues including muscle and pancreatic β-cells 82.
Although initially viewed as an antiobesity hormone, leptin’s primary role is to serve as a metabolic signal of energy sufficiency rather than excess 83. Leptin levels rapidly decline with caloric restriction and weight loss. This decline is associated with adaptive physiological responses to starvation including increased appetite and decreased energy expenditure. These same responses are observed in leptin-deficient mice and humans, despite massive obesity. Furthermore, these responses are readily normalized by low-dose leptin replacement. In contrast, common forms of obesity are characterized by elevated circulating leptin. Neither endogenously high leptin levels nor treatment with exogenous leptin is effective in ameliorating this obesity, consistent with a state of leptin resistance 84, 85. The mechanism for leptin resistance is unknown but may result from defects in leptin signaling or transport across the blood-brain barrier 84, 85. Clearly, the most sensitive portion of the leptin dose-response curve resides in the physiological range between the low levels induced by food restriction and the rising levels induced by refeeding and not in the supraphysiological range associated with obesity. This role of leptin as an indicator of energy sufficiency makes sense from an evolutionary perspective but provides no consolation in our current environment of energy abundance.
In addition to its effects on energy homeostasis, leptin regulates neuroendocrine function and traditional endocrine systems. Leptin deficiency in Lepob/Lepob mice is associated with activation of the hypothalamic-pituitary-adrenal (HPA) axis and suppression of the hypothalamic-pituitary-thyroid and -gonadal axes. Leptin decreases hypercortisolemia in Lepob/Lepob mice, inhibits stress-induced secretion of hypothalamic CRH in mice, and inhibits cortisol secretion from rodent and human adrenocortical cells in vitro. The role of leptin in HPA activity in humans in vivo remains unclear. Leptin also normalizes suppressed thyroid hormone levels in leptin-deficient mice and humans, in part via stimulation of TRH expression and secretion from hypothalamic TRH neurons 84, 86. Leptin accelerates puberty in normal mice and restores normal gonadotropin secretion and reproductive function in leptin-deficient mice and humans 87. Leptin replacement during fasting prevents starvation-induced changes in the hypothalamic-pituitary-gonadal and -thyroid axes in healthy men 88. Leptin also has direct effects via peripheral leptin receptors in the ovary, testis, prostate, and placenta 89.
Several other important endocrine effects of leptin include regulation of immune function, hematopoiesis, angiogenesis, and bone development. Leptin normalizes the suppressed immune function associated with malnutrition and leptin deficiency 90. Leptin also promotes proliferation and differentiation of hematopoietic cells, alters cytokine production by immune cells, stimulates endothelial cell growth and angiogenesis, and accelerates wound healing 89. An important role for leptin in bone development is supported by the observation that leptin-deficient Lepob/Lepob mice have increased bone mass, despite hypercortisolemia and hypogonadism 91. Chemical lesions of specific hypothalamic neurons suggest that the ventral medial hypothalamus (VMH) is involved in leptin’s effect on bone mass (24). Leptin-responsive neurons in the VMH are known to influence sympathetic nervous system (SNS) activity. Indeed, mice with defective SNS activity due to absence of dopamine β-hydroxylase have high bone mass and are resistant to the antiosteogenic effects of leptin, whereas transgenic overexpression of leptin in osteoblasts has no effect on bone mass 91. These data suggest that leptin decreases bone mass indirectly via activation of the SNS 91. Leptin clearly has diverse endocrine function in addition to its effects on energy homeostasis. As a result, leptin is the prototype for all adipose tissue-derived endocrine hormones.
TNFα (Tumour Necrosis Factor alpha)
TNFα is a cytokine initially described as an endotoxin-induced factor causing necrosis of tumors and subsequently shown to be identical to cachexin, a factor secreted by macrophages in vitro 92. TNFα is a 26-kDa transmembrane protein that is cleaved into a 17-kDa biologically active protein that exerts its effects via type I and type II TNFα receptors. Within adipose tissue, TNFα is expressed by adipocytes and stromovascular cells 93. TNFα expression is greater in sc compared with visceral adipose tissue, but this finding may be dependent on total and regional fat mass 94. Adipocytes also express both types of TNFα receptors as membrane bound and soluble forms 92. The ability of TNFα to induce cachexia in vivo naturally led to an extensive evaluation of its role in energy homeostasis.
Although initially suspected of playing a role in cachexia, TNFα has now been implicated in the pathogenesis of obesity and insulin resistance 92, 95, 96. Adipose tissue expression of TNFα is increased in obese rodents and humans and is positively correlated with adiposity and insulin resistance 96, 96, 96. Although circulating concentrations of TNFα are low relative to local tissue concentrations, plasma TNFα levels have been positively correlated with obesity and insulin resistance in some studies but not others 96. Chronic exposure to TNFα induces insulin resistance both in vitro and in vivo (25). Treatment with neutralizing soluble TNFα receptors improves insulin sensitivity in rodent obesity but not in humans 92. Targeted gene deletion of TNFα or its receptors significantly improves insulin sensitivity and circulating nonesterified fatty acids (NEFAs) in rodent obesity 97.
Several potential mechanisms for TNFα’s metabolic effects have been described. First, TNFα influences gene expression in metabolically important tissues such as adipose tissue and liver 98. In adipose tissue, TNFα represses genes involved in uptake and storage of NEFAs and glucose, suppresses genes for transcription factors involved in adipogenesis and lipogenesis, and changes expression of several adipocyte-secreted factors including adiponectin and IL-6 98. In liver, TNFα suppresses expression of genes involved in glucose uptake and metabolism and fatty acid oxidation and increases expression of genes involved in de novo synthesis of cholesterol and fatty acids 98. Second, TNFα impairs insulin signaling. This effect is mediated by activation of serine kinases that increase serine phosphorylation of insulin receptor substrate-1 and −2, making them poor substrates for insulin receptor kinases and increasing their degradation 95. TNFα also impairs insulin signaling indirectly by increasing serum NEFAs, which have independently been shown to induce insulin resistance in multiple tissues 92. Thus, whereas TNFα clearly affects multiple metabolic processes, the relative contribution of direct endocrine effects may be less significant than the indirect effects resulting from autocrine or paracrine modulation of NEFAs or other adipose tissue-derived hormones.
Adiponectin
Adiponectin was independently characterized in 1995 and 1996 by four groups using different methods, hence its alternative names of apM1 (adipose most abundant gene transcript 1), Acrp30 (adipocyte complement-related protein of 30 kDa), adipoQ, and GBP28 (gelatin binding protein of 28 kDa) 99. Adiponectin is highly and specifically expressed in differentiated adipocytes and circulates at high levels in the bloodstream 100. Adiponectin expression is higher in sc than visceral adipose tissue 93.
Adiponectin receptors (AdipoR) 1 and 2 have been identified. The receptors contain seven-transmembrane domains but are structurally and functionally distinct from G protein-coupled receptors. AdipoR1 is expressed primarily in muscle and functions as a high-affinity receptor for globular adiponectin and a low-affinity receptor for full-length adiponectin. AdipoR2 is expressed primarily in liver and functions as an intermediate-affinity receptor for both globular and full-length adiponectin. Thus, the biological effects of adiponectin depend on not only the relative circulating concentrations and properties of the different adiponectin isoforms but also the tissue-specific expression of the adiponectin receptor subtypes.
A strong and consistent inverse association between adiponectin and both insulin resistance and inflammatory states has been established 101. Plasma adiponectin declines before the onset of obesity and insulin resistance in nonhuman primates, suggesting that hypoadiponectinemia contributes to the pathogenesis of these conditions 102. Adiponectin levels are low with insulin resistance due to either obesity or lipodystrophy, and administration of adiponectin improves metabolic parameters in these conditions 100, 103. Conversely, adiponectin levels increase when insulin sensitivity improves, as occurs after weight reduction or treatment with insulin-sensitizing drugs 100. Furthermore, several polymorphisms in the adiponectin gene are associated with obesity and insulin resistance 100. These epidemiological findings are corroborated by studies in murine models with altered adiponectin expression. Adiponectin-deficient mice develop premature diet-induced glucose intolerance and insulin resistance, increased serum NEFAs, and increased vascular neointimal smooth muscle proliferation in response to injury 104. This unfavorable metabolic profile occurs without significant differences in body weight or food intake 104. In contrast, transgenic overexpression of adiponectin in mice leads to improved insulin sensitivity, glucose tolerance, and serum NEFAs 104.
Several mechanisms for adiponectin’s metabolic effects have been described. In the liver, adiponectin enhances insulin sensitivity, decreases influx of NEFAs, increases fatty acid oxidation, and reduces hepatic glucose output. In muscle, adiponectin stimulates glucose use and fatty acid oxidation. Within the vascular wall, adiponectin inhibits monocyte adhesion by decreasing expression of adhesion molecules, inhibits macrophage transformation to foam cells by inhibiting expression of scavenger receptors, and decreases proliferation of migrating smooth muscle cells in response to growth factors. In addition, adiponectin increases nitric oxide production in endothelial cells and stimulate angiogenesis. These effects are mediated via increased phosphorylation of the insulin receptor, activation of AMP-activated protein kinase, and modulation of the nuclear factor κB pathway 104. Taken together, these studies suggest that adiponectin is a unique adipocyte-derived hormone with antidiabetic, antiinflammatory, and antiatherogenic effects.
Macrophages and monocyte chemoattractant protein (MCP)-1
Obesity is associated with increased adipose tissue infiltration by macrophages 105. Activated macrophages secrete inflammatory factors that contribute to insulin resistance, including TNFα and IL-6. MCP-1, a chemokine that recruits monocytes to sites of inflammation, is expressed and secreted by adipose tissue 105. Whereas the cellular source of MCP-1 expression is unclear, both adipocytes and stromovascular cells have been implicated 105. Adipose tissue expression of MCP-1 and circulating MCP-1 levels are increased in rodent obesity, suggesting that MCP-1-mediated macrophage infiltration of adipose tissue may contribute to the metabolic abnormalities associated with obesity and insulin resistance 106, 107.
IL-6
IL-6 is another cytokine associated with obesity and insulin resistance 108. Within adipose tissue, IL-6 and IL-6R are expressed by adipocytes and adipose tissue matrix 93. Expression and secretion of IL-6 are 2 to 3 times greater in visceral relative to sc adipose tissue 93. In contrast to TNFα, IL-6 circulates at high levels in the bloodstream, and as much as one third of circulating IL-6 originates from adipose tissue 108.
Adipose tissue IL-6 expression and circulating IL-6 concentrations are positively correlated with obesity, impaired glucose tolerance, and insulin resistance 108. Both expression and circulating levels decrease with weight loss 108. Furthermore, plasma IL-6 concentrations predict the development of type 2 diabetes and cardiovascular disease 108. Genetic polymorphisms of the IL-6 locus have been linked to obesity, energy expenditure, insulin sensitivity, and type 2 diabetes 108. Furthermore, peripheral administration of IL-6 induces hyperlipidemia, hyperglycemia, and insulin resistance in rodents and humans 108 IL-6 also decreases insulin signaling in peripheral tissues by reducing expression of insulin receptor signaling components and inducing suppressor of cytokine signaling 3 , a negative regulator of both leptin and insulin signaling 109. IL-6 also inhibits adipogenesis and decreases adiponectin secretion 108 These peripheral effects of IL-6 are consistent with the above epidemiological findings, suggesting a causal role for IL-6 in obesity and insulin resistance.
Plasminogen activator inhibitor (PAI)-1
Several proteins of the hemostasis and fibrinolytic system are secreted by adipocytes including tissue factor and PAI-1 110. Plasma PAI-1 levels are elevated in obesity and insulin resistance, are positively correlated with features of the metabolic syndrome, and predict future risk for type 2 diabetes and cardiovascular disease 110, 111. Plasma PAI-1 levels are strongly associated with visceral adiposity, which is independent of other variables including insulin sensitivity, total adipose tissue mass, or age 110. Weight loss and improvement in insulin sensitivity due to treatment with metformin or thiazoladinediones significantly reduce circulating PAI-1 levels 110. TNFα contributes to the elevated PAI-1 levels observed in obesity and insulin resistance 111.
Adipsin and acylation stimulating protein (ASP)
Adipsin (complement factor D) is one of several adipose tissue-derived complement components that are required for the enzymatic production of ASP, a complement protein that affects both lipid and glucose metabolism 112. Although adipsin was initially shown to be decreased in rodent obesity 113, subsequent studies in humans indicate that both adipsin and ASP positively correlate with adiposity, insulin resistance, dyslipidemia, and cardiovascular disease 112. ASP influences lipid and glucose metabolism via several mechanisms 112. ASP promotes fatty acid uptake by increasing lipoprotein lipase activity, promotes triglyceride synthesis by increasing the activity of diacylglycerol acyltransferase, and decreases lipolysis and release of NEFAs from adipocytes 112. ASP also increases glucose transport in adipocytes by increasing the translocation of glucose transporters and enhances glucose-stimulated insulin secretion from pancreatic β-cells 112. Not surprisingly, mice with targeted deletion of complement protein C3 (obligate ASP-deficient) have delayed postprandial clearance of triglycerides and NEFAs. Despite delayed lipid clearance, these mice have decreased body weight and fat mass, improved steady-state serum lipid profiles, and improved glucose tolerance and insulin sensitivity 112. This improved metabolic profile is attributed in part to increased energy expenditure and fatty acid oxidation in liver and muscle 114. A G protein-coupled receptor for ASP, known as C5L2, has been identified and is expressed in adipocytes 115. These findings support an endocrine role for ASP and related complement components in metabolism.
How Are Overweight and Obesity Diagnosed ?
The most common way to find out whether you’re overweight or obese is to figure out your body mass index (BMI). BMI is an estimate of body fat, and it’s a good gauge of your risk for diseases that occur with more body fat.
BMI is calculated from your height and weight. You can use the chart below or the National Heart, Lung, and Blood Institute’s (NHLBI’s) online BMI calculator to figure out your BMI. Or, you health care provider can measure your BMI.
Why is weight control so important ?
According to the National Heart, Lung, and Blood Institute, obesity is often a contributing factor in:
- gallstones,
- arthritis,
- fertility problems,
- sleep apnea and other breathing disorders,
- high triglycerides, high LDL cholesterol, low HDL cholesterol,
- high blood pressure, heart disease, stroke,
- type 2 diabetes, metabolic syndrome,
- some types of cancers. A population-based study using BMI and cancer incidence data from the GLOBOCAN project estimated that, in 2012 in the United States, about 28,000 new cases of cancer in men (3.5%) and 72,000 in women (9.5%) were due to overweight or obesity 116. The percentage of cases attributed to overweight or obesity varied widely for different cancer types but was as high as 54% for gallbladder cancer in women and 44% for esophageal adenocarcinoma in men.
Some recent WHO global estimates follow:
- In 2014, more than 1.9 billion adults aged 18 years and older were overweight. Of these over 600 million adults were obese.
- Overall, about 13% of the world’s adult population (11% of men and 15% of women) were obese in 2014.
- In 2014, 39% of adults aged 18 years and over (38% of men and 40% of women) were overweight.
- The worldwide prevalence of obesity more than doubled between 1980 and 2014.
The prevalence of obesity in US adults will increase from its present level of about 32% to about 50% by 2030, with increased costs of up to US$66 billion per year for treatment of obesity-associated diseases. Furthermore, an estimated 170 million children (aged <18 years) globally were classified as overweight or obese. This estimate includes more than 25% of all children in some countries, more than double the proportions from the start of the epidemic.
*Adults*
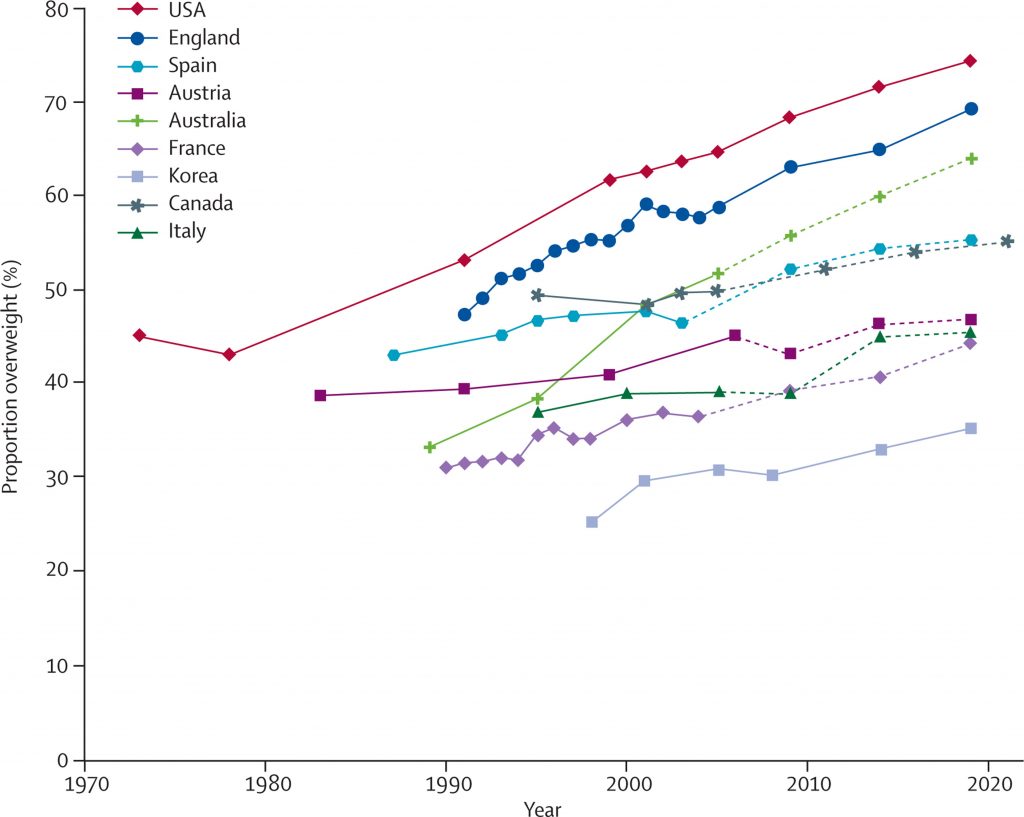
(Source: The Lancet – Reproduced from the Organisation for Economic Co-operation and Development)
Body Mass index (BMI) – the weight in kilograms divided by the square of the height in meters (kg/m2) – is a commonly used index to classify overweight and obesity in adults. However, BMI provides the most useful population-level measure of overweight and obesity as it is the same for both sexes and for all ages of adults. However, it should be considered a rough guide because it may not correspond to the same degree of fatness in different individuals. Measuring BMI while it is a simple, inexpensive method of screening for weight categories, it is not a diagnostic tool. Health professionals use other tools to do further assessments to fully evaluate health risks. These additional tools would include measurements of waist hip ratio, body fat percentage, diet history, exercise patterns, and family history. One thing that experts agree on is that weight is only one factor in our risk for disease. When it comes to evaluating weight and its impact on health, your percentage of body fat, waist circumference, BMI, and physical activity patterns are all important.
Furthermore, BMI does not take into account your age, gender or muscle mass. Nor does it distinguish between lean body mass and fat mass. As a result, some people, such as heavily muscled athletes, may have a high BMI even though they don’t have a high percentage of body fat. In others, such as elderly people, BMI may appear normal even though muscle has been lost with aging.
Fat around your waist is more biologically active and can do more damage to your body than weight around your hips. The research data show that waist circumference is more reliable and more closely correlated with diseases associated with obesity.
According to the National Institutes of Health, a bigger waist circumference (greater than 40 inches for men and 35 inches for women) is linked to a higher risk of type 2 diabetes, high blood pressure, abnormal cholesterol levels, and heart disease when BMI is 25 to 34.9.
BMI is considered an important measure for understanding population trends. For individuals, it is one of many factors that should be considered in evaluating healthy weight, along with waist size, body fat composition, waist circumference, blood pressure, cholesterol level and blood sugar.
The World Health Organization defines overweight as a BMI equal to or more than 25 – 29.9 and obesity as a BMI equal to or more than 30.
Adults with a BMI of 35 or higher and an obesity-related condition (e.g., diabetes) and adults with a BMI of 40 or higher are considered severely obese.
Body Mass Index for Adults
Use the chart below to learn about your BMI. First, find your height (either in inches or centimeters) on the horizontal X-axis. Next, move across the vertical Y-axis to find your weight (either in pounds or kilograms). Weight is measured with underwear but no shoes.
Once you’ve found your weight, move to the very top of that column. This number is your BMI.
Body Mass Index (BMI) is a common measure expressing the relationship (or ratio) of weight to height. The equation is:

What Does Body Mass Index Mean ?
BMI
18.5–24.9 = Normal weight
25.0–29.9 = Overweight
30.0–39.9 = Obese
40.0 and above = Extreme obesity
Although BMI can be used for most men and women, it does have some limits. It may overestimate body fat in athletes and others who have a muscular build. BMI also may underestimate body fat in older people and others who have lost muscle.
**Children**
In 2014, an estimated 41 million children under the age of 5 years were overweight or obese. In Africa, the number of children who are overweight or obese has nearly doubled from 5.4 million in 1990 to 10.6 million in 2014. Nearly half of the children under 5 who were overweight or obese in 2014 lived in Asia.
Body Mass Index for Children and Teens
Overweight are obesity are defined differently for children and teens than for adults. Children are still growing, and boys and girls mature at different rates.
BMIs for children and teens compare their heights and weights against growth charts that take age and sex into account. This is called BMI-for-age percentile. A child or teen’s BMI-for-age percentile shows how his or her BMI compares with other boys and girls of the same age.
For children, age needs to be considered when defining overweight and obesity.
For children under 5 years of age:
- overweight is weight-for-height greater than 2 standard deviations above WHO Child Growth Standards median; and
- obesity is weight-for-height greater than 3 standard deviations above the WHO Child Growth Standards median.
Children aged between 5–19 years
Overweight and obesity are defined as follows for children aged between 5–19 years:
- overweight is BMI-for-age greater than 1 standard deviation above the WHO Growth Reference median; and
- obesity is greater than 2 standard deviations above the WHO Growth Reference median.
What Does the BMI-for-Age Percentile Mean ?
BMI-for-Age Percentile
Less than 5th percentile = Underweight
5th percentile to less than the 85th percentile = Healthy weight
85th percentile to less than the 95th percentile = Risk of overweight
95th percentile or greater =Overweight
For more information about BMI-for-age and growth charts for children, go to the Centers for Disease Control and Prevention’s BMI-for-age calculator.

Causes of Overweight and Obesity
Energy imbalances, some genetic or endocrine medical conditions, and certain medicines are known to cause overweight or obesity 117. The obvious possible drivers of the obesity epidemic are in the food system 2: the increased supply of cheap, palatable, energy-dense foods; improved distribution systems to make food much more accessible and convenient; and more persuasive and pervasive food marketing 118.
Several studies have tested the hypothesis that increases in the food supply are the dominant drivers of the weight gain in populations 119, 120, 121. Results from these investigations show that the rise in food energy supply was more than sufficient to explain the rise in obesity in the USA from the 1970s 119, 120 and most of the weight increase in the UK since the 1980s 121. A related hypothesis is that the policies put in place in the USA and other countries to increase the food supply from the 1970s led to a situation in which the abundance of food in these countries began to push up population energy intake—a reversal of the previous situation in which energy intake was pulled down by decreases in physical activity.
- Energy imbalances cause the body to store fat
Energy imbalances can cause overweight and obesity. An energy imbalance means that your energy IN does not equal your energy OUT. This energy is measured in calories. Energy IN is the amount of calories you get from food and drinks. Energy OUT is the amount of calories that your body uses for things such as breathing, digesting, being physically active, and regulating body temperature.
Overweight and obesity develop over time when you take in more calories than you use, or when energy IN is more than your energy OUT. This type of energy imbalance causes your body to store fat. Any successful intervention targeting obesity (eg, diet, exercise, drugs, bariatric surgery, etc) must tip the balance between energy intake and expenditure.
Data from the US Department of Agriculture lend support to this explanation since they clearly show a reduction of per-person energy available in the food supply from early in the 20th century until the 1960s 122 (see Figure: Food availability for the USA, 1910–2006), mainly because of reduced consumption of wheat products 123, 124. The 1970s saw a striking rise in the quantity of refined carbohydrates and fats in the US food supply 125, 126, which was paralleled by a sharp increase in the available calories (see Figure: Food availability for the USA, 1910–2006) and the onset of the obesity epidemic. There was “a revolution in the mass preparation of food that is roughly comparable to the mass production revolution in manufactured goods that happened a century ago,” that “lowered the time price of food consumption” 2. The increased availability and marketing of cheap, readily available food was so great that food waste has progressively increased by about 50% since the 1970s 119. The period past the 1970’s energy balance flipping point, when energy intake rose because of environmental push factors (i.e, increasingly available, cheap, tasty, highly promoted obesogenic foods). The concomitant rise in weight was the physiological mechanism for restoration of energy balance 127. Food supply data from the US lends support to this flipping point hypothesis, but it needs to be tested in other countries and with more diverse datasets.
Food availability for the USA, 1910–2006 and Energy Balance Flipping Point
Note: There are two distinct phases: a decrease in food energy supply (postulated to be pulled down by reduced energy expenditure requirements for daily living), followed by an increase in food energy supply (postulated to be pushed up by increasing food access). An energy balance flipping point is proposed, marking the change in how the US population generally achieved energy balance.
[Source 122]Failure to suppress caloric intake to offset the calories from calorie-sweetened beverages is part of the mechanism for increased energy intake and the risk for obesity 128, 129, 130. Drinking sugar-sweetened beverages at lunch did not suppress intake of solid food; rather, the calories from beverages added on to total calorie intake 130. For example, drinking apple juice reduced energy intake less than when the same amount of apple was eaten as applesauce or as pieces of apple 129.
It’s important to note that fruit juices are not a better option for weight control than sugar-sweetened beverages. Ounce for ounce, fruit juices-even those that are 100 percent fruit juice, with no added sugar- are as high in sugar and calories as sugary sodas. So it’s no surprise that a recent Harvard School of Public Health study, which tracked the diet and lifestyle habits of 120,000 men and women for up to 20 years, found that people who increased their intake of fruit juice gained more weight over time than people who did not 131. Pediatricians and public health advocates recommend that children and adults limit fruit juice to just a small glass a day, if they consume it at all.
Your body uses certain nutrients such as carbohydrates or sugars, proteins, and fats from the foods you eat to:
- make energy for immediate use to power routine daily body functions and physical activity.
- store energy for future use by your body. Sugars are stored as glycogen in the liver and muscles. Fats are stored mainly as triglycerides in fat tissue.
The amount of energy that your body gets from the food you eat depends on the type of foods you eat, how the food is prepared, and how long it has been since you last ate.
Soft drinks and other sugar-sweetened beverages are the primary sources of added sugars in Americans’ diets, including that of children and adolescents 132, 133, 134, 135. Between 1970 and 2000, per person daily consumption of caloric soft drinks increased 70%, from 7.8 ounces to 13.2 ounces 135.
A 6 year follow up study supported by the National Heart, Lung and Blood Institute published in the American Heart Association journal, Circulation, linked regular consumption of sugar-sweetened drinks (such as sodas and fruit drinks) to increased visceral fat, a type of body fat that may contribute to a higher risk of diabetes and heart disease 136, 137.
In the setting of a pandemic of obesity and related chronic diseases, the American Heart Association recently released a scientific statement recommending reductions in added sugar intake to no more than 100 calories per day for most American women and no more than 150 calories per day for most American men from added sugars 138. The statement identified sugar sweetened beverages as the primary source of added sugars in the American diet 139. While it has long been suspected that sugar sweetened beverages contribute at least in part to the obesity epidemic, only in recent years have large epidemiologic studies been able to substantiate the relationship between sugar sweetened beverage consumption and long-term weight gain, type 2 diabetes and cardiovascular risk. It is thought that sugar sweetened beverages’ contribute to weight gain due to their high added sugar content, low satiety and potential incomplete compensation for total energy leading to increased energy intake 140, 141. In addition, because of their high amounts of rapidly absorbable carbohydrates such as various forms of sugar and high-fructose corn syrup and large quantities consumed, sugar sweetened beverages’ may increase type 2 diabetes mellitus and cardiovascular risk, independent of obesity as a contributor to a high dietary glycemic load leading to inflammation, insulin resistance, and impaired ß-cell function 142. Fructose from any sugar or high fructose corn syrup may also increase blood pressure, and promote accumulation of visceral adiposity, dyslipidemia and ectopic fat deposition due to increased hepatic de novo lipogenesis10. Here, we review temporal patterns in SSB consumption, and clinically relevant effects on obesity, type 2 diabetes and cardiovascular disease risk.
Likewise, total calorie intake has increased by approximately 150-300 calories per day, with half of these calories coming from liquid sources, eg. sugar sweetened beverages 143, 144. Because there has been no concomitant change in physical activity 144, 145, the net result is the well known rise in overweight and obesity that currently plague our nation in epidemic proportions 146.
Positive energy balance with as few as 110-165 calorie surplus daily can yield an excess of ten or more pounds over one year’s time 143, 144. “Supersized” portions in the U.S. have maximized energy content of numerous foods and beverages. From 1977 to 1996, “typical” servings for soda and other sugary beverages have increased by 50 and 68 calories, respectively 147.
Unhealthy eating behaviors
Some unhealthy eating behaviors can increase your risk for overweight and obesity.
- Eating more calories than you use. The amount of calories you need will vary based on your sex, age, and physical activity level. Find out your daily calorie needs or goals with the Body Weight Planner 148.
- Eating too much saturated and trans fats
- Eating foods high in added sugars
High-fructose corn syrup is one of the most widely used food ingredients in nearly all soft drinks, canned jams, breakfast cereals and baked goods. An analysis of food consumption patterns by using US Department of Agriculture food consumption tables from 1967 to 2000. The consumption of high-fructose corn syrup increased > 1000% between 1970 and 1990, far exceeding the changes in intake of any other food or food group 149. High-fructose corn syrup now represents > 40% of caloric sweeteners added to foods and beverages and is the sole caloric sweetener in soft drinks in the United States. Our most conservative estimate of the consumption of high-fructose corn syrup indicates a daily average of 132 kcal for all Americans aged > or = 2 y, and the top 20% of consumers of caloric sweeteners ingest 316 kcal from high-fructose corn syrup/day. The increased use of high-fructose corn syrup in the United States mirrors the rapid increase in obesity 149. The digestion, absorption, and metabolism of fructose differ from those of glucose. Hepatic metabolism of fructose favors de novo lipogenesis 150. In addition, unlike glucose, fructose does not stimulate insulin secretion or enhance leptin production. Because insulin and leptin act as key afferent signals in the regulation of food intake and body weight, this suggests that dietary fructose may contribute to increased energy intake and weight gain. Furthermore, calorically sweetened beverages may enhance caloric overconsumption. Thus, the increase in consumption of high-fructose corn syrup has a temporal relation to the epidemic of obesity, and the overconsumption of high-fructose corn syrup in calorically sweetened beverages may play a role in the epidemic of obesity.
The 2015-2020 Dietary Guidelines for Americans 151 recommends consuming less than 10% of calories per day from added sugars. The guidelines also note that many foods and beverages that contain added sugars also tend to be high in calories and provide few or no important nutrients or dietary fiber.
The majority of sugars in typical American diets are sugars added to foods during processing, preparation, or at the table. These “added sugars” sweeten the flavor of foods and beverages and improve their palatability. They also are added to foods for preservation purposes and to provide functional attributes, such as viscosity, texture, body, and browning capacity.
Although the body’s response to sugars does not depend on whether they are naturally present in food or added to foods, sugars found naturally in foods are part of the food’s total package of nutrients and other healthful components. In contrast, many foods that contain added sugars often supply calories, but few or no essential nutrients and no dietary fiber.
For example, fizzy drinks do not make you feel full as quickly as foods do. This makes them easy to over-consume. And a small fizzy drink contains nine teaspoons of added sugar, so drinking just one can means that you have almost reached your recommended maximum intake for that whole day.
- Lack of physical activity
Lack of physical activity due to high amounts of TV, computer, videogame or other screen usage has been associated with a high body mass index. Healthy lifestyle changes, such as being physically active and reducing screen time, can help you aim for a healthy weight.
- Not enough sleep
Many studies have seen a high BMI in people who do not get enough sleep. Some studies have seen a relationship between sleep and the way our bodies use nutrients for energy and how lack of sleep can affect hormones that control hunger urges.
- High amounts of stress
Acute stress and chronic stress affect the brain and trigger the production of hormones, such as cortisol, that control our energy balances and hunger urges. Acute stress can trigger hormone changes that make you not want to eat. If the stress becomes chronic, hormone changes can make you eat more and store more fat.
- Age
Childhood obesity remains a serious problem in the United States, and some populations are more at risk for childhood obesity than others. The risk of unhealthy weight gain increases as you age. Adults who have a healthy BMI often start to gain weight in young adulthood and continue to gain weight until 60 to 65 years old, when they tend to start losing weight.
- Sex
In the United States, obesity is more common in black or Hispanic women than in black or Hispanic men. A person’s sex may also affect the way the body stores fat. For example, women tend to store less unhealthy fat in the abdomen than men do.
Overweight and obesity is also common in women with polycystic ovary syndrome (PCOS). This is an endocrine condition that causes large ovaries and prevents proper ovulation, which can reduce fertility.
- Unhealthy environments
Many environmental factors can increase your risk for overweight and obesity:
- social factors such as having a low socioeconomic status or an unhealthy social or unsafe environment in the neighborhood
- built environment factors such as easy access to unhealthy fast foods, limited access to recreational facilities or parks, and few safe or easy ways to walk in your neighborhood
- exposure to chemicals known as obesogens that can change hormones and increase fatty tissue in our bodies.
- Race or ethnicity
Overweight and obesity is highly prevalent in some racial and ethnic minority groups. Rates of obesity in American adults are highest in blacks, followed by Hispanics, then whites. This is true for men or women. While Asian men and women have the lowest rates of unhealthy BMIs, they may have high amounts of unhealthy fat in the abdomen. Samoans may be at risk for overweight and obesity because they may carry a DNA variant that is associated with increased BMI but not with common obesity-related complications.
- Genetic syndromes
Several genetic syndromes are associated with overweight and obesity, including the following.
+ Prader-Willi syndrome
+ Bardet-Biedl syndrome
+ Alström syndrome
+ Cohen syndrome
The study of these genetic syndromes has helped researchers understand obesity.
- Endocrine disorders
Because the endocrine system produces hormones that help maintain energy balances in the body, the following endocrine disorders or tumors affecting the endocrine system can cause overweight and obesity.
- Hypothyroidism. People with this condition have low levels of thyroid hormones. These low levels are associated with decreased metabolism and weight gain, even when food intake is reduced. People with hypothyroidism also produce less body heat, have a lower body temperature, and do not efficiently use stored fat for energy.
- Cushing’s syndrome. People with this condition have high levels of glucocorticoids, such as cortisol, in the blood. High cortisol levels make the body feel like it is under chronic stress. As a result, people have an increase in appetite and the body will store more fat. Cushing’s syndrome may develop after taking certain medicines or because the body naturally makes too much cortisol.
- Tumors. Some tumors, such as craneopharingioma, can cause severe obesity because the tumors develop near parts of the brain that control hunger.
- Medicines
Medicines such as antipsychotics, antidepressants, antiepileptics, and antihyperglycemics can cause weight gain and lead to overweight and obesity.
Talk to your doctor if you notice weight gain while you are using one of these medicines. Ask if there are other forms of the same medicine or other medicines that can treat your medical condition, but have less of an effect on your weight. Do not stop taking the medicine without talking to your doctor.
How Does Lack of Sleep Affect Your Body Weight
Researchers speculate that there are several ways that chronic sleep deprivation might lead to weight gain, either by increasing how much food people eat or decreasing the energy that they burn 43.
- Sleep deprivation could increase your energy intake by
Increasing hunger: Sleep deprivation may alter the hormones that control hunger 45. One small study, for example, found that young men who were deprived of sleep had higher levels of the appetite-stimulating hormone ghrelin and lower levels of the satiety-inducing hormone leptin, with a corresponding increase in hunger and appetite-especially for foods rich in fat and carbohydrates 46.
Giving you more time to eat: People who sleep less each night may eat more than people who get a full night’s sleep simply because they have more waking time available 47. Recently, a small laboratory study found that people who were deprived of sleep and surrounded by tasty snacks tended to snack more-especially during the extra hours they were awake at night-than when they had adequate sleep 48.
Prompting you to choose less healthy diets: Observational studies have not seen a consistent link between sleep and food choices 43. But one study of Japanese workers did find that workers who slept fewer than six hours a night were more likely to eat out, have irregular meal patterns, and snack than those who slept more than six hours 49.
- Sleep deprivation could decrease your energy expenditure by
Decreasing your physical activity: People who don’t get enough sleep are more tired during the day, and as a result may curb their physical activity 50. Some studies have found that sleep-deprived people tend to spend more time watching TV, less time playing organized sports, and less time being physically active than people who get enough sleep. But these differences in physical activity or TV viewing are not large enough to explain the association between sleep and weight 51.
Lowering your body temperature: In laboratory experiments, people who are sleep-deprived tend to see a drop in their body temperatures 51. This drop, in turn, may lead to decreased energy expenditure. Yet a recent study did not find any link between sleep duration and total energy expenditure 52.
Sleep Deprivation and Childhood Obesity
Dozens of studies spanning five continents have looked at the link between sleep duration and obesity in children. Most (but not all) have found a convincing association between too little sleep and increased weight 43, 152, 153, 154, 155, 156, 157. The strongest evidence has come from studies that have tracked the sleep habits of large numbers of children over long periods of time (longitudinal studies), and have also adjusted for the many other factors that could increase children’s obesity risk, such as parents’ obesity, television time, and physical activity.
A British study, for example, that followed more than 8,000 children from birth found that those who slept fewer than 10 and a half hours a night at age 3 had a 45 percent higher risk of becoming obese by age 7, compared to children who slept more than 12 hours a night 152. Similarly, Project Viva, a U.S. prospective cohort study of 915 children, found that infants who averaged fewer than 12 hours of sleep a day had twice the odds of being obese at age 3, compared with those who slept for 12 hours or more 155. Maternal depression during pregnancy, introduction of solid foods before the age of 4 months, and infant TV viewing were all associated with shorter sleep duration 158.
Childhood sleep habits may even have a long-term effect on weight, well into adulthood. Researchers in New Zealand followed 1,037 children from birth until age 32, collecting information from parents on the average number of hours their children slept at ages 5, 7, 9, and 11 157. Each one hour reduction in sleep during childhood was associated with a 50 percent higher risk of obesity at age 32.
Keep in mind that these are all observational studies-and even though they suggest an association between sleep and weight, they cannot conclusively show that getting enough sleep lowers children’s risk of obesity. More definitive answers may come from randomized clinical trials that test whether increasing infant or childhood sleep lowers the risk of obesity.
The first such trial to look at the influence of a sleep intervention on childhood obesity, fielded in Australia, enrolled 328 7-month-old infants who already had sleep problems, as reported by their mothers 159. The trial sought to test whether counseling mothers on behavioral techniques to manage infant sleep problems would improve infants’ sleep and also lower mothers’ rates of depression; the researchers followed up at intervals over six years to see if the intervention had any effect on obesity. At one year of age, infants in the intervention group had fewer reported sleep problems than infants in the control group, but there was no difference in sleep duration between the groups; at 2 and 6 years of age, there was no difference in sleep problems or sleep duration between the groups. Thus, it is perhaps no surprise that at 6 years of age, obesity rates were the same in both groups. The study also had serious shortcomings, among them, the fact that 40 percent of the participants had dropped out by the six-year follow-up.
Two large trials currently underway, one in the U.S. and the other in New Zealand, should offer more conclusive evidence on the relationship between sleep duration and obesity 160, 161. Both trials are testing whether teaching parents how to develop good sleep and feeding habits in their newborn infants helps prevent the development of obesity during the toddler years. Early results from the U.S. study have been encouraging 160.
Sleep Deprivation and Adult Obesity
Most studies that measure adults’ sleep habits at one point in time (cross-sectional studies) have found a link between short sleep duration and obesity 43. Longitudinal studies, though, can better answer questions about causality-and in adults the findings from such studies have been less consistent than those in children 162.
The largest and longest study to date on adult sleep habits and weight is the Nurses’ Health Study, which followed 68,000 middle-age American women for up to 16 years 50. Compared to women who slept seven hours a night, women who slept five hours or less were 15 percent more likely to become obese over the course of the study. A similar investigation in the Nurses’ Health Study and the Nurses’ Health Study II, a cohort of younger women, looked at the relationship between working a rotating night shift-an irregular schedule that mixes day and evening work with a few night shifts, throwing off circadian rhythms and impairing sleep-and risk of type 2 diabetes and obesity 163. Researchers found that the longer women worked a rotating night shift, the greater their risk of developing diabetes and obesity.
Other researchers have fielded smaller, shorter longitudinal studies on adult sleep habits and weight in the U.S. and Canada as well as the U.K. and Europe 162. Some have found a link between short sleep duration and obesity, while others have not. Interestingly, a few studies in adults have reported that getting too much sleep is linked to a higher risk of obesity 50, 164. This is most likely due to a phenomenon that researchers call “reverse causation.” People who sleep for longer than normal may have an obesity-related condition that has led to their longer sleep habits-sleep apnea, obstructive lung disease, depression, or cancer, for example-rather than long sleep coming first and then leading to obesity.
An in-process pilot study may provide more answers on whether getting a longer night’s sleep can help with weight loss 165. Researchers are recruiting 150 obese adults who are “short sleepers” (who sleep fewer than 6.5 hours a night) and randomly assigning them to either maintain their current sleep habits or else receive coaching on how to extend their nightly sleep by at least half an hour to an hour. Investigators will track study participants’ sleep habits and weight for three years.
How Does Anxiety and Stress Lead to Obesity
There is much truth behind the phrase “stress eating” 31. Indeed, individuals attempting to lose weight often cite life stressors as reasons for abandoning diet plans, and following strict diet/exercise programs is a stressful endeavour. Rigidly restricting food intake often leads to compensatory overeating and psychological distress associated with diet-breaking may further increase disinhibited eating 32. Researchers have linked weight gain to stress 33 and according to an American Psychological Association survey, about one-fourth of Americans rate their stress level as 8 or more on a 10-point scale 34.
In the short term, stress can shut down appetite. A structure in the brain called the hypothalamus produces corticotropin-releasing hormone, which suppresses appetite 35. The brain also sends messages to the adrenal glands atop the kidneys to pump out the hormone epinephrine (also known as adrenaline). Epinephrine helps trigger the body’s fight-or-flight response, a revved-up physiological state that temporarily puts eating on hold.
But if stress persists, it’s a different story. The adrenal glands release another hormone called cortisol, and cortisol increases appetite and may also ramp up motivation in general, including the motivation to eat 36. Once a stressful episode is over, cortisol levels should fall, but if the stress doesn’t go away — or if a person’s stress response gets stuck in the “on” position — cortisol may stay elevated 37.
Stress also seems to affect food preferences. Numerous studies, granted, many of them in animals — have shown that physical or emotional distress increases the intake of food high in fat, sugar, or both. High cortisol levels, in combination with high insulin levels, may be responsible. Other research suggests that ghrelin, a “hunger hormone,” may have a role 38, 39, 40. Stress-induced increases in ghrelin, which further stimulate appetite, might impede efforts to maintain weight loss. Interventions to prevent increased ghrelin levels in response to stress and weight loss are needed 41 .
Once ingested, fat- and sugar-filled foods seem to have a feedback effect that inhibits activity in the parts of the brain that produce and process stress and related emotions. These foods really are “comfort” foods in that they seem to counteract stress — and this may contribute to people’s stress-induced craving for those foods. Of course, overeating isn’t the only stress-related behavior that can add pounds. Stressed people also lose sleep (sleep deprivation), exercise less, and drink more alcohol, all of which can contribute to excess weight.
Several studies suggest that the peptide hormone ghrelin mediates some of the usual behavioral responses to acute and chronic stress 166. Circulating ghrelin levels have been found to rise following stress. It has been proposed that this elevated ghrelin helps animals cope with stress by generating antidepressant-like behavioral adaptations, although another study suggests that decreasing central nervous system ghrelin expression has antidepressant-like effects. Ghrelin also seems to have effects on anxiety, although these have been shown to be alternatively anxiogenic or anxiolytic. As an example, in a large cross sectional epidemiological U.S. study, a body mass index ≥30 was found to be associated with a 25% higher rate of mood disorders 167, 168. Conversely, other studies suggest that psychological stress can increase the risk of developing obesity. For instance, a longitudinal study found that major depression in late adolescent girls was associated with a 2.3-fold increased risk of obesity in adulthood 169. Also, a review of U.S. veterans with posttraumatic stress disorder showed a significantly increased rate of obesity 170. The mechanisms by which ghrelin affects mood-related behaviors have not yet been fully elucidated, but likely include interaction with its receptors in one or more brain sites critical to mood determination 166.
Ghrelin appears to increase anxiety via its actions on the (corticotropin-releasing hormone) CRH/ACTH (adrenocorticotropin hormone) system. Consistent with animal research, Takaya et al. 171 reported that intravenous injections of synthetic human ghrelin increased ACTH (adrenocorticotropin hormone) and cortisol (often called the stress hormone, cortisol principal steroid hormone produced by the adrenal cortex, regulates carbohydrate metabolism and the immune system and maintains blood pressure). Similarly, Arvat and colleagues 172 found that injecting ghrelin increased ACTH and cortisol.
Using the Trier Social Stress Test (5 min of performing a speech and 5 min of mental calculation in front of three observers), Rouach et al. 40 observed that among participants whose cortisol levels increased in response to stress, ghrelin levels increased. These participants also reported higher stress and anxiety after the TSST. Self-reported urges to eat did not differ between participants in upper and lower quartiles of ghrelin levels. However, the participants who reported an increased urge to overeat after the TSST had marginally higher ghrelin levels than participants who reported no change in urge to overeat. This highlights the importance of individual differences in the ghrelin response to stress.
- Stress Gender differences
Some research suggests a gender difference in stress-coping behavior, with women being more likely to turn to food and men to alcohol or smoking. And a Finnish study that included over 5,000 men and women showed that obesity was associated with stress-related eating in women but not in men.
Harvard researchers 35 have reported that stress from work and other sorts of problems correlates with weight gain, but only in those who were overweight at the beginning of the study period. One theory is that overweight people have elevated insulin levels, and stress-related weight gain is more likely to occur in the presence of high insulin.
How much cortisol people produce in response to stress may also factor into the stress–weight gain equation. In 2007, British researchers designed an ingenious study that showed that people who responded to stress with high cortisol levels in an experimental setting were more likely to snack in response to daily hassles in their regular lives than low-cortisol responders.
Summary Anxiety and Stress Causing Obesity and Weight Gain
There are now plenty of research evidence available on relationships between stress and weight gain in humans. Existing research suggests that injecting ghrelin leads to increased stress hormone secretion. Specific behavioural techniques designed to reduce ghrelin (e.g. increasing sleep and exercise), added to traditional weight loss maintenance programs, might help maintain lower ghrelin levels and appetite while body weight stabilizes. A ghrelin-reduction programme might include stress management techniques such as time management, relaxation (e.g. diaphragmatic breathing, progressive muscle relaxation), cognitive restructuring, meditation and biofeedback 173.
Consequences of Obesity
Consequences of Obesity – Video Part 1
Consequences of Obesity – Video Part 2
Consequences of Obesity – Video Part 3
Consequences of Obesity – Video Part 4
What are common health consequences of overweight and obesity in Adults and Children ?
Cardiovascular Disease (e.g. Coronary Heart Disease, Stroke, Hypertension, High Total Blood Cholesterol) in the US, in 2010 affected about 84 million men and women ages 20 years and older (35% of the population). About 75% of adults who were overweight or obese, had at least one cardiometabolic risk factor (i.e., high blood pressure, abnormal blood lipids, smoking, or diabetes).
About 10% of children ages 8 to 17 years had either borderline hypertension (8%) or hypertension (2%). And 8% of children ages 8 to 17 years had total cholesterol levels ≥200 mg/dL.
Effects of Obesity
- Coronary Heart Disease
As your body mass index rises, so does your risk for coronary heart disease (CHD). Coronary heart disease is a condition in which a waxy substance called plaque builds up inside the coronary arteries. These arteries supply oxygen-rich blood to your heart.
Plaque can narrow or block the coronary arteries and reduce blood flow to the heart muscle. This can cause angina (chest pain or discomfort) or a heart attack.
Cardiovascular diseases (mainly heart disease and stroke), which were the leading cause of death in 2012.
Obesity also can lead to heart failure. This is a serious condition in which your heart can’t pump enough blood to meet your body’s needs.
- High Blood Pressure
Blood pressure is the force of blood pushing against the walls of the arteries as the heart pumps blood. If this pressure rises and stays high over time, it can damage the body in many ways. Your chances of having high blood pressure are greater if you’re overweight or obese.
- Stroke
Being overweight or obese can lead to a buildup of plaque in your arteries. Eventually, an area of plaque can rupture, causing a blood clot to form.
If the clot is close to your brain, it can block the flow of blood and oxygen to your brain and cause a stroke. The risk of having a stroke rises as BMI increases.
- Type 2 Diabetes
Adult Diabetes (Type 2 Diabetes) is a disease in which the body’s blood glucose, or blood sugar, level is too high. Normally, the body breaks down food into glucose and then carries it to cells throughout the body. The cells use a hormone called insulin to turn the glucose into energy.
In type 2 diabetes, the body’s cells don’t use insulin properly. At first, the body reacts by making more insulin. Over time, however, the body can’t make enough insulin to control its blood sugar level.
Diabetes is a leading cause of early death, CHD, stroke, kidney disease, and blindness. Most people who have type 2 diabetes are overweight.
- Abnormal Blood Fats
If you’re overweight or obese, you’re at increased risk of having abnormal levels of blood fats. These include high levels of triglycerides and LDL (“bad”) cholesterol and low levels of HDL (“good”) cholesterol.
Abnormal levels of these blood fats are a risk factor for coronary heart disease. For more information about triglycerides and LDL and HDL cholesterol, go to the Health Topics High Blood Cholesterol article.
- Metabolic Syndrome
Metabolic syndrome is the name for a group of risk factors that raises your risk for heart disease and other health problems, such as diabetes and stroke.
You can develop any one of these risk factors by itself, but they tend to occur together. A diagnosis of metabolic syndrome is made if you have at least three of the following risk factors:
- A large waistline. This is called abdominal obesity or “having an apple shape.” Having extra fat in the waist area is a greater risk factor for coronary heart disease than having extra fat in other parts of the body, such as on the hips.
- A higher than normal triglyceride level (or you’re on medicine to treat high triglycerides).
- A lower than normal HDL cholesterol level (or you’re on medicine to treat low HDL cholesterol).
- Higher than normal blood pressure (or you’re on medicine to treat high blood pressure).
- Higher than normal fasting blood sugar (or you’re on medicine to treat diabetes).
- Cancer
Being overweight or obese raises your risk for colon, breast, ovarian, endometrial, liver and gallbladder and kidney and prostate cancers. See the section below “What is known about the relationship between obesity and cancer ?”
- Osteoarthritis
Osteoarthritis is a common joint problem of the knees, hips, and lower back. The condition occurs if the tissue that protects the joints wears away. Extra weight can put more pressure and wear on joints, causing pain.
- Sleep Apnea
Sleep apnea is a common disorder in which you have one or more pauses in breathing or shallow breaths while you sleep.
A person who has sleep apnea may have more fat stored around the neck. This can narrow the airway, making it hard to breathe.
- Obesity Hypoventilation Syndrome
Obesity hypoventilation syndrome is a breathing disorder that affects some obese people. In Obesity hypoventilation syndrome, poor breathing results in too much carbon dioxide (hypoventilation) and too little oxygen in the blood (hypoxemia).
Obesity hypoventilation syndrome can lead to serious health problems and may even cause death.
- Reproductive Problems
Obesity can cause menstrual issues and infertility in women.
- Gallstones
Gallstones are hard pieces of stone-like material that form in the gallbladder. They’re mostly made of cholesterol. Gallstones can cause stomach or back pain.
People who are overweight or obese are at increased risk of having gallstones. Also, being overweight may result in an enlarged gallbladder that doesn’t work well.
- Non-Alcoholic Fatty Liver Disease (NAFLD) and Non-Alcoholic Steatohepatitis (NASH);
The prevalence of Non-Alcoholic Fatty Liver Disease (NAFLD) is increasingly placing a greater burden on healthcare resources. The rate of progression of Non-Alcoholic Fatty Liver Disease is variable; being overweight and having diabetes are associated with an increased risk of progressive disease. The average age of people with Non-Alcoholic Steatohepatitis (NASH) where there is excess fat with inflammation and damage in the liver, is 40–50 years and for non-alcoholic steatohepatitis-cirrhosis (excess fat with inflammation and scarring of the liver), 50–60 years. However, the emerging epidemic of childhood obesity means that increasing numbers of younger people have non-alcoholic fatty liver disease, with some prevalence studies showing that up to 38% of obese children have evidence of Non-Alcoholic Fatty Liver Disease. With non-alcoholic fatty liver disease progressing through its spectrum even in childhood, the age that people develop significant liver disease is likely to fall and early diagnosis and management are therefore important issues at all ages.
Globally, 44% of diabetes, 23% of ischaemic heart disease and 7–41% of certain cancers are attributable to overweight and obesity. The risk for these noncommunicable diseases increases with increases in BMI.
What is known about the relationship between obesity and cancer ?
Nearly all of the evidence linking obesity to cancer risk comes from large cohort studies, a type of observational study. However, data from observational studies can be difficult to interpret and cannot definitively establish that obesity causes cancer. That is because obese or overweight people may differ from lean people in ways other than their body fat, and it is possible that these other differences—rather than their body fat—are what explains their different cancer risk.
Despite the limitations of the study designs, there is consistent evidence that higher amounts of body fat are associated with increased risks of a number of cancers 174, including:
- Endometrial cancer: Obese and overweight women are two to about four times as likely as normal-weight women to develop endometrial cancer (cancer of the lining of the uterus), and extremely obese women are about seven times as likely to develop the more common of the two main types of this cancer 175. The risk of endometrial cancer increases with increasing weight gain in adulthood, particularly among women who have never used menopausal hormone therapy 176.
- Esophageal adenocarcinoma: People who are overweight or obese are about twice as likely as normal-weight people to develop a type of esophageal cancer called esophageal adenocarcinoma, and people who are extremely obese are more than four times as likely 177.
- Gastric cardia cancer: People who are obese are nearly twice as likely as normal-weight people to develop cancer in the upper part of the stomach, that is, the part that is closest to the esophagus 178.
- Liver cancer: People who are overweight or obese are up to twice as likely as normal-weight people to develop liver cancer. The association between overweight/obesity and liver cancer is stronger in men than women 179, 180.
- Kidney cancer: People who are overweight or obese are nearly twice as likely as normal-weight people to develop renal cell cancer, the most common form of kidney cancer 181. The association of renal cell cancer with obesity is independent of its association with high blood pressure, a known risk factor for kidney cancer 182.
- Multiple myeloma: Compared with normal-weight individuals, overweight and obese individuals have a slight (10% to 20%) increase in the risk of developing multiple myeloma 183.
- Meningioma: The risk of this slow-growing brain tumor that arises in the membranes surrounding the brain and the spinal cord is increased by about 50% in people who are obese and about 20% in people who are overweight 184.
- Pancreatic cancer: People who are overweight or obese are about 1.5 times as likely to develop pancreatic cancer as normal-weight people 185.
- Colorectal cancer: People who are obese are slightly (about 30%) more likely to develop colorectal cancer than normal-weight people 186. A higher BMI is associated with increased risks of colon and rectal cancers in both men and in women, but the increases are higher in men than in women 186.
- Gallbladder cancer: Compared with normal-weight people, people who are overweight have a slight (about 20%) increase in risk of gallbladder cancer, and people who are obese have a 60% increase in risk of gallbladder cancer 187, 188. The risk increase is greater in women than men.
- Breast cancer: Many studies have shown that, in postmenopausal women, a higher BMI is associated with a modest increase in risk of breast cancer. For example, a 5-unit increase in BMI is associated with a 12% increase in risk 189. Among postmenopausal women, those who are obese have a 20% to 40% increase in risk of developing breast cancer compared with normal-weight women 190. The higher risks are seen mainly in women who have never used menopausal hormone therapy and for tumors that express hormone receptors. Obesity is also a risk factor for breast cancer in men 191. In premenopausal women, by contrast, overweight and obesity have been found to be associated with a 20% decreased risk of breast tumors that express hormone receptors 190.
- Ovarian cancer: Higher BMI is associated with a slight increase in the risk of ovarian cancer, particularly in women who have never used menopausal hormone therapy 192. For example, a 5-unit increase in BMI is associated with a 10% increase in risk among women who have never used menopausal hormone therapy 192.
- Thyroid cancer: Higher BMI (specifically, a 5-unit increase in BMI) is associated with a slight (10%) increase in the risk of thyroid cancer 193.
Does avoiding weight gain or losing weight decrease the risk of cancer ?
Fewer studies have examined possible associations between weight loss and cancer risk. Some of these have found decreased risks of breast, endometrial, colon, and prostate cancers among people who have lost weight. However, most of these studies were not able to evaluate whether the weight loss was intentional or unintentional (and possibly related to underlying health problems).
Many observational studies have provided consistent evidence that people who have lower weight gain during adulthood have lower risks of colon cancer, kidney cancer, and—for postmenopausal women—breast, endometrial, and ovarian cancers 194.
Stronger evidence for a relationship between weight loss and cancer risk comes from studies of people who have undergone bariatric surgery (surgery performed on the stomach or intestines to induce weight loss). Obese people who have bariatric surgery appear to have lower risks of obesity-related cancers than obese people who do not have bariatric surgery 195.
Nevertheless, the follow-up study of weight and breast cancer in the Women’s Health Initiative 196 found that for women who were already overweight or obese at baseline, weight change (either gain or loss) was not associated with breast cancer risk during follow-up. However, for women who were of normal weight at baseline, gaining more than 5% of body weight was associated with increased breast cancer risk.
How does obesity affect cancer survivorship ?
Most of the evidence about obesity in cancer survivors comes from people who were diagnosed with breast, prostate, or colorectal cancer. Research indicates that obesity may worsen several aspects of cancer survivorship, including quality of life, cancer recurrence, cancer progression, and prognosis (survival) 197, 198.
For example, obesity is associated with increased risks of treatment-related lymphedema in breast cancer survivors 199 and incontinence in prostate cancer survivors treated with radical prostatectomy 200. In a large clinical trial of patients with stage II and stage III rectal cancer, those with a higher baseline BMI (particularly men) had an increased risk of local recurrence 201. Death from multiple myeloma is 50% more likely for people at the highest levels of obesity compared with people at normal weight 202.
Several randomized clinical trials in breast cancer survivors have reported weight loss interventions that resulted in both weight loss and beneficial changes in biomarkers that have been linked to the association between obesity and prognosis 203, 204. However, there is little evidence about whether weight loss improves cancer recurrence or prognosis 205.
What research is being done on obesity and cancer ?
Several areas of research are exploring mechanisms that link obesity and cancer 206, 207. One research area involves understanding the role of the microbes that live in the human gastrointestinal tract (collectively called the gut microbiota, or microbiome) in both type 2 diabetes and obesity. Both conditions are associated with dysbiosis, an imbalance in the collection of these microbes. For example, the gut microbiomes of obese people are different from, and less diverse than, those of non-obese people. Imbalances in the gut microbiota are associated with inflammation, altered metabolism, and genotoxicity, which may in turn be related to cancer. Experiments in mice show that the microbiome may influence the efficacy of some types of cancer treatment, particular immunotherapy 208, 209. Researchers are beginning to think about ways to change the microbiota of cancer patients to improve their outcomes.
Another area of investigation is the role of insulin receptor signaling in cancer. Many cancer cells express elevated levels of IR-A, a form of the insulin receptor that has a high affinity for insulin and related growth factors. Researchers are investigating how these factors contribute to metabolic disease and cancer and which may be useful targets for therapeutic interventions to prevent obesity-related cancers.
Researchers are also trying to understand why the association between obesity and the risks of some cancers vary among racial/ethnic groups. For example, obesity has been found to be more strongly associated with an increased risk of prostate cancer among African American men than among white men 210. This observation might reflect a difference in the biological effects of obesity between these two groups, such as a difference in the effects of obesity on inflammation or insulin secretion.
What are common health consequences of overweight and obesity in Children and Teens ?
Childhood and adolescent obesity is one of the leading global public health concerns. In the past 30 years, obesity has tripled among school-aged children and teens. Rapid weight gain in children as young as six years old has increased globally, and has significant mental and physical health consequences such as diabetes, high blood pressure, asthma, sleep problems, and low self-esteem. Obesity in childhood and adolescence can persist into adulthood, increasing the risk of poor health in later life.
Evidence 211 shows that, if left unchecked, overweight and obesity persists into adulthood. Adult obesity can often lead to type 2 diabetes, heart disease, stroke, some cancers, mental health problems, reduced quality of life and shorter life expectancy.
According to the National Health and Nutrition Examination Survey 2009–2010, about 1 in 6 American children ages 2–19 are obese. The survey also suggests that overweight and obesity are having a greater effect on minority groups, including Blacks and Hispanics.
Overweight and obesity also increase the health risks for children and teens. Type 2 diabetes once was rare in American children, but an increasing number of children are developing the disease.
Also, overweight children are more likely to become overweight or obese as adults, with the same disease risks.
- Type 2 Diabetes 212. This chronic condition affects the way your child’s body uses sugar (glucose). Obesity and a sedentary lifestyle increase the risk of type 2 diabetes.
- High Blood Pressure (Hypertension) 213, 212. Compared with normal weight children, systolic blood pressure was higher by 4.54 mm Hg in overweight children and by 7.49 mm Hg in obese children. Obese children had a significant increase in left ventricular mass of 19.12 g, compared with normal weight children.
- Metabolic syndrome 214, 215. This cluster of conditions can put your child at risk of heart disease, diabetes or other health problems. Conditions include high blood pressure, high blood sugar, high triglycerides, low HDL (“good”) cholesterol and excess abdominal fat.
- High Cholesterol 212. Obesity adversely affected concentrations of all blood lipids; total cholesterol and triglycerides were 0.15 mmol/L and 0.26 mmol/L higher in obese children, respectively. Moreover, a poor diet can also cause your child to develop one or both of these conditions. These factors can contribute to the buildup of plaques in the arteries. These plaques can cause arteries to narrow and harden, which can lead to a heart attack or stroke later in life.
- Asthma 216. Overweight and especially obese children are at increased risk of subsequent physician diagnosed asthma in comparison to normal weight children.
- Sleep disorders – Obstructive Sleep Apnea 217, 218, 219. Obstructive sleep apnea is a potentially serious disorder in which a child’s breathing repeatedly stops and starts during sleep. Obstructive sleep apnea significantly complicates obesity and is an independent risk factor for cardiovascular, metabolic, neuro-cognitive burden as well as negative impact on the quality of life in obese children.
- Nonalcoholic fatty liver disease (NAFLD). This disorder, which usually causes no symptoms, causes fatty deposits to build up in the liver. NAFLD can lead to scarring and liver damage.
- Insulin Resistance (Prediabetes) 212. Fasting insulin and insulin resistance were significantly higher in obese participants but not in overweight participants.
Social and emotional complications 220
- Low self-esteem and being bullied. Children often tease or bully their overweight peers, who suffer a loss of self-esteem and an increased risk of depression as a result.
- Behavior and learning problems. Overweight children tend to have more anxiety and poorer social skills than normal-weight children do. These problems might lead children who are overweight to act out and disrupt their classrooms at one extreme, or to withdraw socially at the other.
- Depression. Low self-esteem can create overwhelming feelings of hopelessness, which can lead to depression in some children who are overweight.
The strong prejudice in this country against obese persons is evident in children as young as 6 years of age. There is discrimination against obese persons in both academic and work settings. Despite this discrimination, overweight persons in the general population show no greater psychological disturbance than do non-obese persons.
Why is weight control so important ?
According to the National Heart, Lung, and Blood Institute, obesity is often a contributing factor in:
- gallstones,
- arthritis,
- fertility problems,
- sleep apnea and other breathing disorders,
- high triglycerides, high LDL cholesterol, low HDL cholesterol,
- high blood pressure, heart disease, stroke,
- type 2 diabetes, metabolic syndrome,
- some types of cancers.
Compounded by an ageing population, in the next two decades, extrapolation of the historic trend in the USA would project an increase in annual medical cost from treating obesity-related disorders of US$28 billion per year by 2020 and $66 (19–112) billion per year by 2030.
Policies in education, food processing, distribution and marketing influence children’s dietary habits and preferences as well as their physical activity patterns. Increasingly, these influences are promoting unhealthy weight gain leading to a steady rise in the prevalence of childhood obesity.
Childhood obesity is associated with a higher chance of obesity, premature death and disability in adulthood. But in addition to increased future risks, obese children experience breathing difficulties, increased risk of fractures, hypertension, early markers of cardiovascular disease, insulin resistance and psychological effects.
Making Changes to Diet, Physical Activity and Behaviour may Reduce Obesity in Children and Adolescents
An increasing number of children and adolescents across the world are too heavy for their age, height and sex. Obese children are at increased risk of diabetes, high blood pressure, asthma and sleep problems. As well as physical health problems, studies have also shown that being overweight or obese in this age group is associated with low self-esteem, which can lead to mental health problems and poor quality of life 221.
Fortunately, new evidence reveals that there are proven, effective ways to tackle overweight and obesity in the young. Two reviews 222, 223, published today, show that combinations of diet, exercise and behavioural change produce small but important reductions in measures of body mass in school-aged children and adolescents.
Latest health evidence looking at lifestyle interventions in children aged six to eleven years olds 222 and adolescents aged 12 to 17 years olds 223, may reduce obesity in children and adolescents 222, 223.
Two new Cochrane Reviews published 22 June 2017, summarized the results of 114 studies which involved more than 13,000 children and young people. They show that a combination of diet, physical activity, and behavioural change interventions may reduce weight in children aged six to 11 years and in adolescents aged 12 to 17. The two reviews 222, 223 look at the effects of diet, physical activity, and behavioural interventions in treating children with overweight or obesity from six years old to early adulthood. They are the last two reviews in a series of six that covers surgery, drug therapy, interventions targeting parents only, and lifestyle interventions for children of pre-school age.
Both these latest Cochrane Reviews 222, 223 will inform ongoing work by the World Health Organization.
The childhood review looks at evidence from 70 studies conducted in over 8,000 six to 11-year-olds from Europe, the USA, Canada, New Zealand, Australia, Japan, and Malaysia. Most studies compared behaviour-changing interventions with no treatment or usual care. The majority of trials (65/70) involved both the child and their parents or caregivers.
The quality of the evidence was low but suggests that compared to no treatment or usual care, interventions incorporating combinations of diet, physical activity, and behaviour change may have a small, short-term effect in reducing children’s weight and body mass index (a proxy measure of body fat based on weight in relation to height, sex, and age). The researchers know less about the effects of diet, physical activity, and behaviour change on self-esteem and quality of life, because few of the trials looked at these outcomes.
The review of adolescents found 44 completed studies including just under 5,000 young people with overweight or obesity aged between 12 to 17 years. Fifty additional studies are still ongoing and have not yet reported their results. Most studies assessed the combined effects of diet, physical activity, and behavioural change interventions, but there was variation in the content and duration of the interventions and their delivery, and the comparators used. There was moderate-quality evidence that combinations of diet, physical activity, and behaviour change reduce an adolescent’s weight by about three and a half kilos, and low-quality evidence that these interventions may reduce body mass index by just over 1 kg/m2. These effects were maintained in longer term trials which lasted for up to two years. The findings from this review also suggest a moderately improved quality of life, but did not find firm evidence of an advantage or disadvantage for improving young people’s self-esteem, physical activity, and food intake.
Dr Emma Mead, who led the six-to-11-year-old review 222 as part of her PhD at the School for Health and Social Care, at Teesside University, UK, says these findings complete a very complex picture on a globally important health topic: “These reviews are important because they provide the most up-to-date evidence to show that behaviour changing interventions can help treat children with overweight and obesity. However, we need to do more work to understand how to maintain the positive effects of the intervention after it has finished, and understand which interventions work best in lower income countries, and for families from different socio-demographic backgrounds.”
Dr Lena Al-Khudairy, Research Fellow from the Division of Health Sciences at the University of Warwick, UK, who led the review of adolescents 223, said: “Approaches that combine several interventions can be effective to tackle overweight and obesity in teenagers, but we still need to know more about what specific components are most effective and in whom, and importantly learn more about adolescents’ views about the interventions.”
Why The Need Reduce Obesity
Worldwide about half of all adults have one or more preventable, diet-related chronic diseases, including cardiovascular disease, type 2 diabetes, and overweight and obesity. However, a large body of evidence now shows that healthy eating patterns and regular physical activity can help people achieve and maintain good health and reduce the risk of chronic disease throughout all stages of their lifespan.
If you’re trying to slim down, in addition to healthy eating, exercise is an important part of a healthy way for you to lose pounds. Moderate physical activity can help you burn more calories. And the good news is you don’t need to train for a marathon to get in shape. But perhaps more importantly, regular exercise does so much more than that — no matter the size of your waistline.
Your history of poor eating (poor nutrition) and your lack of physical activity have a cumulative effect on your health and have contributed to significant malnutrition and physical inactivity related health challenges that you are now facing. This is because your poor nutrition and your health are closely related.
From the Facts on Nutrition and Physical Activity on Obesity (shown in a table above), describing the high rates of poor nutrition and physical activity-related chronic diseases and their related risk factors. Abdominal obesity, as measured by waist circumference, is defined as a waist circumference of >102 centimeters in men and >88 centimeters in women.
- Coronary Heart Disease, cardiovascular disease affected about 84 million men and women ages 20 years and older (35% of the population of the US).
- Stroke
- Hypertension, rates of hypertension, abnormal blood lipid profiles, and diabetes are higher in adults with abdominal obesity.
- High Total Blood Cholesterol, nearly three-fourths of those who were overweight or obese, had at least one cardiometabolic risk factor (i.e., high blood pressure, abnormal blood lipids, smoking, or diabetes).
- Diabetes, more than 90% of total diabetes in adults is type 2 diabetes and among children with type 2 diabetes, about 80% were obese.
- Breast Cancer is the third leading cause of cancer death in the United States.
- Colorectal Cancer is the second leading cause of cancer death in the United States.
- Osteoporosis
These diseases affect all ages—- children, adolescents, adults, and older adults—though rates vary by several factors, including race/ethnicity, income status, and body weight status.
Concurrent with these diet-related health problems persisting at high levels, trends in food intake over time show that, at the population level, globally people are not consuming healthy eating patterns. For example, the prevalence of overweight and obesity has risen and remained high for the past 25 years, while healthy eating index scores (total scores are out of 100 possible points), a measure of how food choices align with the Healthy Dietary Guidelines, have remained low (57.8). A score of 100 indicates that recommendations on average healthy eating were met or exceeded. A higher total score indicates a higher quality diet.
Similarly, physical activity levels have remained low over time, with only 21% of adults meeting the Physical Activity Guidelines (Aerobic and Muscle-Strengthening Recommendations).
The Problem Is The SUGAR in Your Diet
Added sugars should only make up not more than 10% of the calories the average person eats in a day. The recommendation to limit intake of calories from added sugars to less than 10 percent per day is a target based on food pattern modeling and national data on intakes of calories from added sugars that demonstrate the need to limit calories from added sugars to meet food group and nutrient needs within calorie limits.

But about one in 10 people get a whopping one-quarter or more of their calories from added sugar. Over the course of the 15-year study on added sugar and heart disease, participants who took in 25% or more of their daily calories as sugar were more than twice as likely to die from heart disease as those whose diets included less than 10% added sugar. Overall, the odds of dying from heart disease rose in tandem with the percentage of sugar in the diet and that was true regardless of a person’s age, sex, physical activity level, and body-mass index (a measure of weight).
Although the evidence for added sugars and health outcomes is still developing, the recommendation to limit calories from added sugars is consistent with research examining eating patterns and health. Strong evidence from mostly prospective cohort studies but also randomized controlled trials has shown that eating patterns that include lower intake of sources of added sugars are associated with reduced risk of cardiovascular disease (e.g. stroke, heart attack, high blood pressure, high blood cholesterol) in adults, and moderate evidence indicates that these eating patterns are associated with reduced risk of obesity, type 2 diabetes, and some types of cancer in adults. As described in another post on healthy eating, eating patterns consist of multiple, interacting food components, and the relationships to health exist for the overall eating pattern. Moderate evidence indicates a relationship between added sugars and dental caries in children and adults.
Obesity treatment
Much of the results on how to lose weight (reduce obesity) and maintaining your weight loss comes from a long-term project known as the National Weight Control Registry (NWCR). Many participants were able to maintain weight loss of at least 30 pounds for at least one year.
The National Weight Control Registry project where some 10,000 people who have lost weight and successfully kept it off for many years have been tracked. Some of their strategies included:
- Getting lots of exercise
- Eating breakfast
- Watching little television
- Keeping a food diary
- Weighing themselves regularly
Most participants reported weighing themselves at least once a week, and just over a third make weighing a daily practice. Over time, people who weighed themselves less often tended to regain more weight than those who increased their weight-monitoring frequency. Researchers speculate that this habit allows people to detect a small weight gain and take action before the problem escalates.
Getting lots of exercise
The table below lists the calories burned by doing dozens of activities listed by category (such as gym activities, training and sports activities, home repair etc.) for 30 minutes. Activities and exercises include walking (casual, race, and everything in between), swimming, jogging, yoga, and even watching TV and sleeping. In each category, activities are listed from least to most calories burned.
| Gym Activities | 125-pound person | 155-pound person | 185-pound person |
| Weight Lifting: general | 90 | 112 | 133 |
| Aerobics: water | 120 | 149 | 178 |
| Stretching, Hatha Yoga | 120 | 149 | 178 |
| Calisthenics: moderate | 135 | 167 | 200 |
| Riders: general | 150 | 186 | 222 |
| Aerobics: low impact | 165 | 205 | 244 |
| Stair Step Machine: general | 180 | 223 | 266 |
| Teaching aerobics | 180 | 223 | 266 |
| Weight Lifting: vigorous | 180 | 223 | 266 |
| Aerobics, Step: low impact | 210 | 260 | 311 |
| Aerobics: high impact | 210 | 260 | 311 |
| Bicycling, Stationary: moderate | 210 | 260 | 311 |
| Rowing, Stationary: moderate | 210 | 260 | 311 |
| Calisthenics: vigorous | 240 | 298 | 355 |
| Circuit Training: general | 240 | 298 | 355 |
| Rowing, Stationary: vigorous | 255 | 316 | 377 |
| Elliptical Trainer: general | 270 | 335 | 400 |
| Ski Machine: general | 285 | 353 | 422 |
| Aerobics, Step: high impact | 300 | 372 | 444 |
| Bicycling, Stationary: vigorous | 315 | 391 | 466 |
| Training and Sport Activities | |||
| Billiards | 75 | 93 | 111 |
| Bowling | 90 | 112 | 133 |
| Dancing: slow, waltz, foxtrot | 90 | 112 | 133 |
| Frisbee | 90 | 112 | 133 |
| Volleyball: non-competitive, general play | 90 | 112 | 133 |
| Water Volleyball | 90 | 112 | 133 |
| Archery: non-hunting | 105 | 130 | 155 |
| Golf: using cart | 105 | 130 | 155 |
| Hang Gliding | 105 | 130 | 155 |
| Curling | 120 | 149 | 178 |
| Gymnastics: general | 120 | 149 | 178 |
| Horseback Riding: general | 120 | 149 | 178 |
| Tai Chi | 120 | 149 | 178 |
| Volleyball: competitive, gymnasium play | 120 | 149 | 178 |
| Walking: 3.5 mph (17 min/mi) | 120 | 149 | 178 |
| Badminton: general | 135 | 167 | 200 |
| Walking: 4 mph (15 min/mi) | 135 | 167 | 200 |
| Kayaking | 150 | 186 | 222 |
| Skateboarding | 150 | 186 | 222 |
| Snorkeling | 150 | 186 | 222 |
| Softball: general play | 150 | 186 | 222 |
| Walking: 4.5 mph (13 min/mi) | 150 | 186 | 222 |
| Whitewater: rafting, kayaking | 150 | 186 | 222 |
| Dancing: disco, ballroom, square | 165 | 205 | 244 |
| Golf: carrying clubs | 165 | 205 | 244 |
| Dancing: Fast, ballet, twist | 180 | 223 | 266 |
| Fencing | 180 | 223 | 266 |
| Hiking: cross-country | 180 | 223 | 266 |
| Skiing: downhill | 180 | 223 | 266 |
| Swimming: general | 180 | 223 | 266 |
| Walk/Jog: jog <10 min. | 180 | 223 | 266 |
| Water Skiing | 180 | 223 | 266 |
| Wrestling | 180 | 223 | 266 |
| Basketball: wheelchair | 195 | 242 | 289 |
| Race Walking | 195 | 242 | 289 |
| Ice Skating: general | 210 | 260 | 311 |
| Racquetball: casual, general | 210 | 260 | 311 |
| Rollerblade Skating | 210 | 260 | 311 |
| Scuba or skin diving | 210 | 260 | 311 |
| Sledding, luge, toboggan | 210 | 260 | 311 |
| Soccer: general | 210 | 260 | 311 |
| Tennis: general | 210 | 260 | 311 |
| Basketball: playing a game | 240 | 298 | 355 |
| Bicycling: 12-13.9 mph | 240 | 298 | 355 |
| Football: touch, flag, general | 240 | 298 | 355 |
| Hockey: field & ice | 240 | 298 | 355 |
| Rock Climbing: rappelling | 240 | 298 | 355 |
| Running: 5 mph (12 min/mile) | 240 | 298 | 355 |
| Running: pushing wheelchair, marathon wheeling | 240 | 298 | 355 |
| Skiing: cross-country | 240 | 298 | 355 |
| Snow Shoeing | 240 | 298 | 355 |
| Swimming: backstroke | 240 | 298 | 355 |
| Volleyball: beach | 240 | 298 | 355 |
| Bicycling: BMX or mountain | 255 | 316 | 377 |
| Boxing: sparring | 270 | 335 | 400 |
| Football: competitive | 270 | 335 | 400 |
| Orienteering | 270 | 335 | 400 |
| Running: 5.2 mph (11.5 min/mile) | 270 | 335 | 400 |
| Running: cross-country | 270 | 335 | 400 |
| Bicycling: 14-15.9 mph | 300 | 372 | 444 |
| Martial Arts: judo, karate, kickbox | 300 | 372 | 444 |
| Racquetball: competitive | 300 | 372 | 444 |
| Rope Jumping | 300 | 372 | 444 |
| Running: 6 mph (10 min/mile) | 300 | 372 | 444 |
| Swimming: breaststroke | 300 | 372 | 444 |
| Swimming: laps, vigorous | 300 | 372 | 444 |
| Swimming: treading, vigorous | 300 | 372 | 444 |
| Water Polo | 300 | 372 | 444 |
| Rock Climbing: ascending | 330 | 409 | 488 |
| Running: 6.7 mph (9 min/mile) | 330 | 409 | 488 |
| Swimming: butterfly | 330 | 409 | 488 |
| Swimming: crawl | 330 | 409 | 488 |
| Bicycling: 16-19 mph | 360 | 446 | 533 |
| Handball: general | 360 | 446 | 533 |
| Running: 7.5 mph (8 min/mile) | 375 | 465 | 555 |
| Running: 8.6 mph (7 min/mile) | 435 | 539 | 644 |
| Bicycling: > 20 mph | 495 | 614 | 733 |
| Running: 10 mph (6 min/mile) | 495 | 614 | 733 |
| Outdoor Activities | |||
| Planting seedlings, shrubs | 120 | 149 | 178 |
| Raking Lawn | 120 | 149 | 178 |
| Sacking grass or leaves | 120 | 149 | 178 |
| Gardening: general | 135 | 167 | 200 |
| Mowing Lawn: push, power | 135 | 167 | 200 |
| Operate Snow Blower: walking | 135 | 167 | 200 |
| Plant trees | 135 | 167 | 200 |
| Gardening: weeding | 139 | 172 | 205 |
| Carrying & stacking wood | 150 | 186 | 222 |
| Digging, spading dirt | 150 | 186 | 222 |
| Laying sod / crushed rock | 150 | 186 | 222 |
| Mowing Lawn: push, hand | 165 | 205 | 244 |
| Chopping & splitting wood | 180 | 223 | 266 |
| Shoveling Snow: by hand | 180 | 223 | 266 |
| Home & Daily Life Activities | |||
| Sleeping | 19 | 23 | 28 |
| Watching TV | 23 | 28 | 33 |
| Reading: sitting | 34 | 42 | 50 |
| Standing in line | 38 | 47 | 56 |
| Cooking | 75 | 93 | 111 |
| Child-care: bathing, feeding, etc. | 105 | 130 | 155 |
| Food Shopping: with cart | 105 | 130 | 155 |
| Moving: unpacking | 105 | 130 | 155 |
| Playing w/kids: moderate effort | 120 | 149 | 178 |
| Heavy Cleaning: wash car, windows | 135 | 167 | 200 |
| Child games: hop-scotch, jacks, etc. | 150 | 186 | 222 |
| Playing w/kids: vigorous effort | 150 | 186 | 222 |
| Moving: household furniture | 180 | 223 | 266 |
| Moving: carrying boxes | 210 | 260 | 311 |
| Home Repair | |||
| Auto Repair | 90 | 112 | 133 |
| Wiring and Plumbing | 90 | 112 | 133 |
| Carpentry: refinish furniture | 135 | 167 | 200 |
| Lay or remove carpet/tile | 135 | 167 | 200 |
| Paint, paper, remodel: inside | 135 | 167 | 200 |
| Cleaning rain gutters | 150 | 186 | 222 |
| Hanging storm windows | 150 | 186 | 222 |
| Paint house: outside | 150 | 186 | 222 |
| Carpentry: outside | 180 | 223 | 266 |
| Roofing | 180 | 223 | 266 |
| Occupational Activities | |||
| Computer Work | 41 | 51 | 61 |
| Light Office Work | 45 | 56 | 67 |
| Sitting in Meetings | 49 | 60 | 72 |
| Desk Work | 53 | 65 | 78 |
| Sitting in Class | 53 | 65 | 78 |
| Truck Driving: sitting | 60 | 74 | 89 |
| Bartending/Server | 75 | 93 | 111 |
| Heavy Equip. Operator | 75 | 93 | 111 |
| Police Officer | 75 | 93 | 111 |
| Theater Work | 90 | 112 | 133 |
| Welding | 90 | 112 | 133 |
| Carpentry Work | 105 | 130 | 155 |
| Coaching Sports | 120 | 149 | 178 |
| Masseur, standing | 120 | 149 | 178 |
| Construction, general | 165 | 205 | 244 |
| Coal Mining | 180 | 223 | 266 |
| Horse Grooming | 180 | 223 | 266 |
| Masonry | 210 | 260 | 311 |
| Forestry, general | 240 | 298 | 355 |
| Heavy Tools, not power | 240 | 298 | 355 |
| Steel Mill: general | 240 | 298 | 355 |
| Firefighting | 360 | 446 | 533 |
(Source: Harvard Heart Letter 2004)
The Department of Health and Human Services recommends that healthy adults include aerobic exercise and strength training in their fitness plans, specifically:
- At least 150 minutes of moderate aerobic activity or 75 minutes of vigorous aerobic activity a week, or an equivalent combination of moderate and vigorous aerobic activity
- Strength training exercises of all the major muscle groups at least twice a week
Sustained physical activity is most helpful in the prevention of weight regain. In addition, exercise has a benefit of reducing risks of cardiovascular disease and diabetes, beyond that produced by weight reduction alone.
- Regular exercise has been proven to help you to reduce the risk of chronic illnesses such as heart disease, certain cancers, type 2 diabetes and stroke.
- Regular exercise can help strengthen your bones and muscles.
- Research shows that physical activity can also boost self-esteem, mood, sleep quality and energy.
Start exercising slowly, and gradually increase the intensity. Trying too hard at first can lead to injury.
How Much Activity Do People Need to Prevent Weight Gain?
Weight gain during adulthood can increase the risk of heart disease, diabetes, and other chronic conditions. Since it’s hard for people to lose weight and keep it off, it’s better to prevent weight gain in the first place. Encouragingly, there’s strong evidence that staying active can help people slow down or stave off “middle-age spread” 224. The more active people are, the more likely they are to keep their weight steady 225; the more sedentary, the more likely they are to gain weight over time 226. But it’s still a matter of debate exactly how much activity people need to avoid gaining weight. The latest evidence suggests that the recommended two and a half hours a week may not be enough.
The Women’s Health Study, for example, followed 34,000 middle-age women for 13 years to see how much physical activity they needed to stay within 5 pounds of their weight at the start of the study. Researchers found that women in the normal weight range at the start needed the equivalent of an hour a day of moderate-to-vigorous physical activity to maintain a steady weight 227.
Vigorous activities seem to be more effective for weight control than slow walking 228, 229. The Nurses’ Health Study II 230, for example, followed more than 18,000 women for 16 years to study the relationship between changes in physical activity and weight. Although women gained, on average, about 20 pounds over the course of the study, those who increased their physical activity by 30 minutes per day gained less weight than women whose activity levels stayed steady. And the type of activity made a difference: Bicycling and brisk walking helped women avoid weight gain, but slow walking did not.
How Much Activity Do People Need to Lose Weight?
Exercise can help promote weight loss, but it seems to work best when combined with a lower calorie eating plan 231. If people don’t curb their calories, however, they likely need to exercise for long periods of time-or at a high intensity-to lose weight 232, 233.
In one study 232, for example, researchers randomly assigned 175 overweight, inactive adults to either a control group that did not receive any exercise instruction or to one of three exercise regimens-low intensity (equivalent to walking 12 miles/week), medium intensity (equivalent to jogging 12 miles/week), or high intensity (equivalent to jogging 20 miles per week). All study volunteers were asked to stick to their usual diets. After six months, those assigned to the high-intensity regimen lost abdominal fat, whereas those assigned to the low- and medium-intensity exercise regimens had no change in abdominal fat 232.
More recently, researchers conducted a similar trial with 320 post-menopausal women 234, randomly assigning them to either 45 minutes of moderate-to-vigorous aerobic activity, five days a week, or to a control group. Most of the women were overweight or obese at the start of the study. After one year, the exercisers had significant decreases in body weight, body fat, and abdominal fat, compared to the non-exercisers 234.
The Bottom Line
- For Weight Control, Aim for an Hour of Activity a Day
Being moderately active for at least 30 minutes a day on most days of the week can help lower the risk of chronic disease. But to stay at a healthy weight, or to lose weight, most people will need more physical activity-at least an hour a day-to counteract the effects of increasingly sedentary lifestyles, as well as the strong societal influences that encourage overeating.
Keep in mind that staying active is not purely an individual choice: The so-called “built environment”-buildings, neighborhoods, transportation systems, and other human-made elements of the landscape-influences how active people are 235. People are more prone to be active, for example, if they live near parks or playgrounds, in neighborhoods with sidewalks or bike paths, or close enough to work, school, or shopping to safely travel by bike or on foot. People are less likely to be active if they live in sprawling suburbs designed for driving or in neighborhoods without recreation opportunities.
Local and state governments wield several policy tools for shaping people’s physical surroundings, such as planning, zoning, and other regulations, as well as setting budget priorities for transportation and infrastructure. Strategies to create safe, active environments include curbing traffic to make walking and cycling safer, building schools and shops within walking distance of neighborhoods, and improving public transportation, to name a few. Such changes are essential to make physical activity an integral and natural part of people’s everyday lives-and ultimately, to turn around the obesity epidemic.
How much exercise do you need for general good health?
For general good health, the 2008 Physical Activity Guidelines for Americans 236 recommends that adults get a minimum of 2-1/2 hours per week of moderate-intensity aerobic activity. Yet many people may need more than 2-1/2 hours of moderate intensity activity a week to stay at a stable weight 236.
The Women’s Health Study 237, for example, followed 34,000 middle-aged women for 13 years to see just how much physical activity they needed to stay within 5 pounds of their weight at the start of the study. Researchers found that women who were in the normal weight range at the start of the study needed the equivalent of an hour a day of physical activity to stay at a steady weight 237.
If you are exercising mainly to lose weight, 60 minutes or so a day may be effective in conjunction with a healthy diet 238.
If you currently don’t exercise and aren’t very active during the day, any increase in exercise or physical activity is good for you.
Aerobic physical activity—any activity that causes a noticeable increase in your heart rate—is especially beneficial for disease prevention.
Some studies show that walking briskly for even one to two hours a week (15 to 20 minutes a day) starts to decrease the chances of having a heart attack or stroke, developing diabetes, or dying prematurely.
You can combine moderate and vigorous exercise over the course of the week, and it’s fine to break up your activity into smaller bursts as long as you sustain the activity for at least 10 minutes.
Exercise Intensity:
Moderate-intensity aerobic activity is any activity that causes a slight but noticeable increase in breathing and heart rate. One way to gauge moderate activity is with the “talk test”—exercising hard enough to break a sweat but not so hard you can’t comfortably carry on a conversation.
Vigorous-intensity aerobic activity causes more rapid breathing and a greater increase in heart rate, but you should still be able to carry on a conversation—with shorter sentences.
Here is a summary of the 2008 Physical Activity Guidelines for Americans 236
Children and adolescents should get at least 1 hour or more a day of physical activity in age-appropriate activities, spending most of that engaged in moderate- or vigorous–intensity aerobic activities. They should partake in vigorous-intensity aerobic activity on at least three days of the week, and include muscle-strengthening and bone strengthening activities on at least three days of the week.
Healthy adults should get a minimum of 2-1/2 hours per week of moderate-intensity aerobic activity, or a minimum of 1-1/4 hours per week of vigorous-intensity aerobic activity, or a combination of the two. That could mean a brisk walk for 30 minutes a day, five days a week; a high-intensity spinning class one day for 45 minutes, plus a half hour jog another day; or some other combination of moderate and vigorous activity. Doubling the amount of activity (5 hours moderate- or 2-1/2 hours vigorous-intensity aerobic activity) provides even more health benefits. Adults should also aim to do muscle-strengthening activities at least two days a week.
Healthy older Adults should follow the guidelines for healthy adults. Older adults who cannot meet the guidelines for healthy adults because of chronic conditions should be as physically active as their abilities and conditions allow. People who have chronic conditions such as arthritis and type 2 diabetes should talk to a healthcare provider about the amount and type of activity that is best. Physical activity can help people manage chronic conditions, as long as the activities that individuals choose match their fitness level and abilities. Even just an hour a week of activity has health benefits. Older adults who are at risk of falling should include activities that promote balance.
Strength training for all ages
Studies have shown strength training to increase lean body mass, decrease fat mass, and increase resting metabolic rate (a measurement of the amount of calories burned per day) in adults 239. While strength training on its own typically does not lead to weight loss 236, its beneficial effects on body composition may make it easier to manage one’s weight and ultimately reduce the risk of disease, by slowing the gain of fat—especially abdominal fat 240.
- Muscle is metabolically active tissue; it utilizes calories to work, repair, and refuel itself. Fat, on the other hand, doesn’t use as much energy. We slowly lose muscle as part of the natural aging process, which means that the amount of calories we need each day starts to decrease, and it becomes easier to gain weight.
- Strength training regularly helps preserve lean muscle tissue and can even rebuild some that has been lost already.
- Weight training has also been shown to help fight osteoporosis. For example, a study in postmenopausal women examined whether regular strength training and high-impact aerobics sessions would help prevent osteoporosis. Researchers found that the women who participated in at least two sessions a week for three years were able to preserve bone mineral density at the spine and hip; over the same time period, a sedentary control group showed bone mineral density losses of 2 to 8 percent 241.
- In older populations, resistance training can help maintain the ability to perform functional tasks such as walking, rising from a chair, climbing stairs, and even carrying one’s own groceries. An emerging area of research suggests that muscular strength and fitness may also be important to reducing the risk of chronic disease and mortality, but more research is needed 242, 243.
- A systematic review of 8 studies 244 examining the effects of weight-bearing and resistance-based exercises on the bone mineral density in older men found resistance training to be an effective strategy for preventing osteoporosis in this population. Resistance training was found to have more positive effects on bone mineral density than walking, which has a lower impact 244.
The Physical Activity Guidelines for Americans recommends that muscle strengthening activities be done at least two days a week 236. Different types of strength training activities are best for different age groups.
- When talking about the benefits of exercise, keeping the heart and blood vessels healthy usually gets most of the attention. For many individuals, though, stretching and strength training exercises may be just as important.
- Strength training, also known as resistance training, weight training, or muscle-strengthening activity, is one of the most beneficial components of a fitness program.
Children and Adolescents: Choose unstructured activities rather than weight lifting exercises 236.
Examples:
- Playing on playground equipment
- Climbing trees
- Playing tug-of-war
Active Adults: Weight training is a familiar example, but there are other options 236:
- Calisthenics that use body weight for resistance (such as push-ups, pull-ups, and sit-ups)
- Carrying heavy loads
- Heavy gardening (such as digging or hoeing)
Older Adults: The guidelines for older adults are similar to those for adults; older adults who have chronic conditions should consult with a health care provider to set their activity goals. Muscle strengthening activities in this age group include the following 236:
- Digging, lifting, and carrying as part of gardening
- Carrying groceries
- Some yoga and tai chi exercises
- Strength exercises done as part of a rehab program or physical therapy
Flexibility training
Flexibility training or stretching exercise is another important part of overall fitness. It may help older adults preserve the range of motion they need to perform daily tasks and other physical activities 245.
- The American Heart Association 239 recommends that healthy adults engage in flexibility training two to three days per week, stretching major muscle and tendon groups.
- For older adults, the American Heart Association and American College of Sports Medicine recommend two days a week of flexibility training, in sessions at least 10 minutes long 245. Older adults who are at risk of falling should also do exercises to improve their balance.
Diet
There’s no single rule that applies to everyone, but to lose weight at a safe and sustainable rate of 0.5 to 1kg (1lb to 2lbs) a week, most people are advised to reduce their energy intake by 600 calories a day.
For most men, this will mean consuming no more than 1,900 calories a day, and for most women, no more than 1,400 calories a day.
The best way to achieve this is to swap unhealthy and high-energy food choices – such as fast food, processed food and sugary drinks (including alcohol) – for healthier choices.
Research has also shown that the more freedom people have in planning their weight-loss programs, the greater their prospects for weight loss success. One study suggested that people who followed a calorie-restricted diet regained an average of nine pounds, but those who ate what they wanted—within healthy eating guidelines—regained less than half that amount.
Another study compared two groups of people: those who had lost at least 10% of their weight and kept it off for five years and who were now at a normal weight, and those who were overweight and who had a history of dieting. Researchers found that people in the first group had fewer televisions and fewer high-fat foods at home compared with the second group. The weight-loss maintainers also exercised more, perhaps because they were more likely to have exercise equipment in their homes.
A healthy eating pattern includes:
- A variety of vegetables from all of the subgroups—dark green, red and orange, legumes (beans and peas), starchy, and other
- Fruits, especially whole fruits
- Grains, at least half of which are whole grains
- Fat-free or low-fat dairy, including milk, yogurt, cheese, and/or fortified soy beverages
- A variety of protein foods, including seafood, lean meats and poultry, eggs, legumes (beans and peas), and nuts, seeds, and soy products
- Oils
Try to avoid foods containing high levels of salt because they can raise your blood pressure, which can be dangerous for people who are already obese.
A healthy eating pattern limits:
- Saturated fats and trans fats, added sugars, and sodium (salt)
- Consume less than 10 percent of calories per day from added sugars
- Consume less than 10 percent of calories per day from saturated fats
- Consume less than 2,300 milligrams (mg) per day of sodium
- If alcohol is consumed, it should be consumed in moderation—up to one drink per day for women and up to two drinks per day for men and only by adults of legal drinking age.
- In tandem with the recommendations above, all ages—children, adolescents, adults, and older adults—should meet the healthy exercise to help promote health and reduce the risk of chronic disease. The relationship between your diet and your level of physical activity contribute to the calorie balance and managing your body weight.
You’ll also need to check calorie information for each type of food and drink you consume to make sure you don’t go over your daily limit.
Some restaurants, cafés and fast food outlets provide calorie information per portion, although providing this information isn’t compulsory. Be careful when eating out because some foods can quickly take you over the limit, such as burgers, fried chicken, and some curries or Chinese dishes.
Low Calorie Diets and Weight Loss
In a study published in The New England Journal of Medicine in 2009, followed 811 overweight adults over 2 years 246, who were prescribed Low Calorie Diets (a deficit of 750 kcal per day from baseline, as calculated from the person’s resting energy expenditure and activity level) and all the low calorie diets should include 8% or less of saturated fat, at least 20 g of dietary fiber per day, and 150 mg or less of cholesterol per 1000 kcal). Carbohydrate-rich foods with a low glycemic index were recommended in each diet. Each participant’s caloric prescription represented one of the four diets:
- Fat 20%, Protein 15% and Carbohydrate 65% (Low-fat and Average-protein and High carb)
- Fat 20%, Protein 25% and Carbohydrate 55% (Low-fat and High-protein and Average carb)
- Fat 40%, Protein 15% and Carbohydrate 45% (High-fat and Average-protein and Average carb)
- Fat 40%, Protein 25% and Carbohydrate 35% (High-fat and High-protein and Low carb)
- All participants’ goal for physical activity was 90 minutes of moderate exercise per week. Participation in exercise was monitored by questionnaire and by the online self-monitoring tool.
Group sessions were held once a week, 3 of every 4 weeks during the first 6 months and 2 of every 4 weeks from 6 months to 2 years; individual sessions were held every 8 weeks for the entire 2 years. Daily meal plans in 2-week blocks were provided. Participants were instructed to record their food and beverage intake in a daily food diary and in a web-based self-monitoring tool that provided information on how closely their daily food intake met the goals for macronutrients and energy. Behavioral counseling was integrated into the group and individual sessions to promote adherence to the assigned diets. Contact among the groups was avoided.
At 6 months, participants assigned to each diet had lost an average of 6 kg, which represented 7% of their initial weight; they began to regain weight after 12 months.
- By 2 years, weight loss remained similar in those who were assigned to a diet with 15% protein and those assigned to a diet with 25% protein (3.0 and 3.6 kg, respectively); in those assigned to a diet with 20% fat and those assigned to a diet with 40% fat (3.3 kg for both groups); and in those assigned to a diet with 65% carbohydrates and those assigned to a diet with 35% carbohydrates (2.9 and 3.4 kg, respectively).
- Among the 80% of participants who completed the trial, the average weight loss was 4 kg; 14 to 15% of the participants had a reduction of at least 10% of their initial body weight. Satiety, hunger, satisfaction with the diet, and attendance at group sessions were similar for all diets; attendance was strongly associated with weight loss (0.2 kg per session attended). The diets improved lipid-related risk factors and fasting insulin levels.
Conclusions: Reduced-calorie diets result in clinically meaningful weight loss regardless of which macronutrients they emphasize. All of the diets resulted in meaningful weight loss, despite the differences in macronutrient composition 246.
The study also found that the more group counseling sessions participants attended, the more weight they lost, and the less weight they regained. This supports the idea that not only is what you eat important, but behavioral, psychological, and social factors are important for weight loss as well 246.
Top diets review
With so many diet options to choose from, it can be hard to find a weight loss plan to suit you.
Paleo diet
The paleo diet, also known as the caveman diet, consists of foods that can be hunted and fished – such as meat and seafood – or gathered – such as eggs, nuts, seeds, fruits, vegetables, herbs and spices.
It’s a regime based on the supposed eating habits of our hunter-gatherer ancestors during the Paleolithic era, before the development of agriculture, around 10,000 years ago. That means cereal grains including wheat, dairy, refined sugar, potatoes – as well as anything processed or with added salt – are strictly off the menu.
There’s no official “paleo diet”, but it’s generally seen as a low-carb, high-protein diet, with some variations on carbohydrate and meat intake.
Advocates say the paleo diet is a long-term healthy eating plan that can help you lose weight and reduce your risk of diabetes, heart disease, cancer and other health problems.
Most studies on the paleo-type diet are small, and more long-term research is needed to show conclusively whether or not it’s as effective as some people claim.
A 2015 review 247 of current studies found some moderate evidence for short-term health improvements and weight loss. It concluded that while the modest carbohydrate, healthier fats and lower salt were beneficial, it was less clear whether the restriction on wholegrain foods and dairy was beneficial.
Pros
The paleo diet encourages you to eat less processed food, less high-fat and high-sugar foods (such as cakes, biscuits, crisps), and more fruit and vegetables. Reducing your consumption of high-calorie foods will reduce your calorie intake and help you lose weight.
The diet is simple and doesn’t involve calorie counting. Some plans are more flexible, which can make the diet easier to stick to and increase your chances of success.
Cons
There are no accurate records of the diet of our Stone Age ancestors, so the paleo diet is largely based on educated guesses, and its health claims lack any scientific evidence.
Most versions of the diet encourage eating a lot of meat, which runs counter to current health advice on meat consumption. Many versions ban dairy products and wholegrains, which form part of a healthy, balanced diet. Unless it’s for a medical reason, there’s no need to cut out whole food groups from your diet. Cutting out food groups without careful substitution can lead to nutritional deficiencies.
The paleo diet can be expensive. For example, it advocates eating only grass-fed meat.
Dieticians verdict
Most versions of the paleo diet exclude key food groups, raising the potential for nutritional deficiencies unless careful substitutions are made, and dietary supplements may be necessary.
The diet has some positive aspects, so an adapted version that doesn’t ban any food groups – such as wholegrains, dairy and legumes – would be a better choice.
The diet lacks variety, so there’s a risk you’ll get bored quickly and give up. If you want to copy your paleolithic ancestors, you’re better off mimicking their activity levels rather than their alleged diet.
New Atkins diet
The Atkins diet promises to turn your body into a fat-burning machine. The theory is that by starving yourself of carbohydrates, your body will start burning fat for energy. During the first phase of the diet, designed for rapid weight loss, you’re on a protein-rich diet, with no restrictions on fat, and a daily carb allowance of 20 to 25g.
During the next 3 phases, the weight loss is likely to be more gradual, and regular exercise is encouraged. More carbs are introduced to your diet with the aim of working out what your ideal carb intake is to maintain a healthy weight for life.
Phase 1 is designed to help you lose up to 15lb in 2 weeks, reducing to 2 to 3lb during phase 2.
Pros
You can lose weight very quickly, which can be motivating.
The diet also encourages people to cut out most processed carbs and alcohol. With its diet of red meat, butter, cream, cheese and mayonnaise, it’s one of the diets that appeals most to men.
Cons
Initial side effects can include bad breath, a dry mouth, tiredness, dizziness, insomnia, nausea and constipation from cutting out carbs, and potential for lower fibre intake.
The high intake of saturated fat may increase your risk of heart disease, and there are concerns about the recommendation to add salt.
Dieticians verdict
The amount of processed meat, red meat and saturated fat in this type of diet is an issue, as is the advice to add salt. These all contradict current health advice.
Some could still find it complicated and time-consuming, but the promise of initial rapid weight loss may appeal to and motivate some.
5:2 diet
The 5:2 diet is based on a principle known as intermittent fasting (IF), where you eat normally for 5 days a week and fast on the other 2.
Evidence on the effectiveness of the 5:2 diet is limited when compared with other types of weight loss methods.
One 2013 study compared women placed on a 5:2 diet to those on a Mediterranean diet. More people lost more than 5% of their weight on the 5:2 diet, and body fat loss and insulin sensitivity was better on the 5:2 diet.
However, the weight loss for the 5:2 diet and the Mediterranean diet was similar overall.
Pros
Sticking to a regimen for 2 days a week can be more achievable than 7 days, so you may be more likely to persevere with this way of eating and successfully lose weight.
Two days a week on a restricted diet can lead to greater reductions in body fat, insulin resistance and other chronic diseases.
Cons
The non-restricted days don’t mean unlimited feasting. While you don’t need to be as strict about your calorie consumption, you still need to make healthy choices and be physically active.
There’s a risk that your restricted eating days may not be nutritionally balanced.
Skipping meals could make you feel dizzy, irritable, give you headaches, and make it hard to concentrate, which can affect work and other daily tasks.
Other reported side effects are difficulties sleeping and daytime sleepiness, bad breath and dehydration.
Dieticians verdict
The 5:2 is a simple way to reduce calorie intake. There are lots of versions of this diet, with some being less safe than others.
Many studies on intermittent fasting are short term, involve small numbers of subjects or are animal-based.
If you choose to follow this diet, choose an evidence-backed plan based on healthy, balanced eating and written by a dietitian, such as the “2-Day Diet”.
It’s vital for your health to avoid nutritional deficiencies, dehydration and overeating on non-fasting days.
Never attempt to delay or skip meals if you’re pregnant, or have had or are prone to eating disorders or diabetes.
Dukan diet
The Dukan diet is a low-carb, high-protein diet. There’s no limit to how much you can eat during the plan’s 4 phases, provided you stick to the rules of the plan.
During phase 1, you’re on a strict lean protein diet. This is based on a list of 72 reasonably low-fat, protein-rich foods such as chicken, turkey, eggs, fish and fat-free dairy. This is for an average of 5 days to achieve quick weight loss. Carbs are off limits, except for a small amount of oat bran.
Unlike the Atkins diet, Dukan’s phase 1 bans vegetables and seriously restricts fat. The next 3 phases of the plan see the gradual introduction of some fruit, veg and carbs, and eventually all foods.
The aim is gradual weight loss of up to 2lb a week and to promote long-term weight management. There’s no time limit to the final phase, which involves having a protein-only day once a week and taking regular exercise.
Pros
You can lose weight very quickly, which can be motivating.
It’s a very strict and prescriptive diet, which some people like. It’s easy to follow, and you don’t need to weigh food or count calories.
Apart from keeping to low-fat, low-salt and high-protein foods, there’s no restriction on how much you can eat during your first 2 weeks.
Cons
At the start of the diet, you may experience side effects such as bad breath, a dry mouth, tiredness, dizziness, insomnia and nausea from cutting out carbs.
The lack of wholegrains, fruit and veg in the early stages of the diet could cause problems such as constipation.
Dieticians verdict
Rapid weight loss can be motivating, but it’s unsustainable and unhealthy. The Dukan diet isn’t nutritionally balanced, which is acknowledged by the fact you need a vitamin supplement and a fibre top-up in the form of oat bran.
There’s a danger this type of diet could increase your risk of long-term health problems if you don’t stick to the rules. The diet lacks variety in the initial phases, so there’s a risk you’ll get bored quickly and give up.
South Beach Diet
The South Beach Diet is a low-GI (glycaemic index) diet originally developed for heart patients in the US. There’s no calorie counting and no limits on portions. You’re encouraged to eat 3 meals and 2 snacks a day, and follow an exercise plan. People who have more than 10lb to lose start with phase 1.
This is a 2-week rapid weight loss regime where you eat lean protein, including meat, fish and poultry, as well as some low-GI vegetables and unsaturated fats. Low-GI carbs are reintroduced during phases 2 and 3, which encourage gradual and sustainable weight loss.
Pros
If you can avoid phase 1 and start on phase 2, there are fewer dietary restrictions in the rest of the plan than some other popular diets.
After phase 1, the diet broadly follows the basic principles of healthy eating. No major food groups are eliminated, and plenty of fruit, veg and low-GI carbs are recommended.
Cons
The severe dietary restrictions of phase 1 may leave you feeling weak, and you’ll miss out on some vitamins, minerals and fibre. You may initially experience side effects such as bad breath, a dry mouth, tiredness, dizziness, insomnia, nausea and constipation.
Dieticians verdict
The first 2 weeks are the most difficult to get through. We’re concerned this diet promises such significant weight loss – up to 13lb – in the first 2 weeks. But this won’t be all fat: some of the weight loss will include water and carbs, both of which will be replaced when you begin eating more normally.
Once you get past the initial phase, the diet follows the basic principles of healthy eating and should provide the nutrients you need to stay healthy.
Alkaline diet
The alkaline diet is based on the idea that modern diets cause our body to produce too much acid. The theory is that excess acid in the body is turned into fat, leading to weight gain.
High acidity levels have also been blamed on conditions such as arthritis, osteoporosis, tiredness, and kidney and liver disorders. There is however no scientific evidence for this.
The diet involves cutting back on acid-producing foods such as meat, wheat and other grains, refined sugar, dairy products, caffeine, alcohol and processed foods in favour of “alkaline foods”, which reduce the body’s acidity levels. This translates into plenty of fruit and vegetables. The idea is that an alkaline diet helps maintain the body’s acidity at healthy levels.
There is no evidence that you can change your body’s blood acidity (pH level) through what you eat. The weight loss observed among followers is more likely to be the result of eating plenty of fruit and vegetables, and cutting down on sugar, alcohol and processed foods, which is standard healthy weight loss advice.
Pros
The diet contains plenty of good healthy eating advice, such as cutting down on meat, avoiding sugar, alcohol and processed foods, and eating more fruit and veg, nuts, seeds and legumes. This means you’ll be cutting out foods you may normally eat and replacing them with healthier choices, which will also reduce your calorie intake.
Cons
Your body regulates its acidity levels, regardless of diet. When cutting down on dairy products such as milk, cheese and yoghurt, you need to find other calcium substitutes, as cutting out an entire food group is never a good idea.
Getting to grips with what you can and can’t eat on the diet can be time-consuming, particularly in the beginning.
Dieticians verdict
The theory of the alkaline diet is that eating certain foods can help maintain the body’s ideal acidity levels to improve overall health. But your body carefully maintains its pH balance (called homeostasis) regardless of the food you eat.
The diet is not supported by any evidence. Any weight loss is likely to be because you are being careful about what you are eating, reducing high-fat and high-sugar foods as well as overall calories.
Slimming World diet
Slimming World’s weight loss plan encourages you to swap high-fat foods for naturally filling low-fat ones.
You choose your food from a list of low-fat foods they call “Free Foods” that are generally filling and low in energy, such as fruit, vegetables, pasta, potatoes, rice, lean meat, fish and eggs. These can be eaten in unlimited amounts. There are additional healthy extras, such as milk, cheese, cereals and wholemeal bread. There’s no calorie counting, no foods are banned and you’re still allowed the occasional treat.
You can get support from fellow slimmers at weekly group meetings and follow an exercise plan to become gradually more active. The plan is designed to help you lose 1 to 2lb a week. You can also join an online programme.
Pros
No foods are banned, so meals offer balance and variety, and are family-friendly. There is one main plan, called Extra Easy, which is flexible.
The “Body Magic” booklet provided offers ideas to help you raise your activity levels, and meeting as a group can provide valuable support.
Cons
The programme advocates eating plenty of low-energy and filling foods. However, while following the Free Foods list, you may choose to eat more lean protein foods and starchy carbohydrate foods than recommended, as these are unrestricted, and this would limit any weight loss if you went over your daily calorie requirements.
Higher-energy treat foods are still allowed but in small quantities. They are known as “syns”, which is short for synergy but the similarity to the word “sin” won’t be lost on anyone.
Dieticians verdict
The group meetings encourage members to share successes, ideas and recipes with each other, but they may not appeal to everyone. The web-based programme may be helpful for others.
The list of low-energy, filling foods can help to promote a healthy, varied and balanced diet including plenty of fruit and vegetables.
Members gain an appreciation of which foods are higher in energy and should therefore be limited. This is helpful for long-term healthy eating.
SlimFast diet
The SlimFast diet is a low-calorie meal-replacement plan for people with a BMI of 25 and over. It uses SlimFast’s range of products. The plan recommends 3 snacks a day from an extensive list (including crisps and chocolate), 2 meal-replacement shakes or bars, and 1 regular meal taken from a list of recipes on the SlimFast website.
You can stay on the diet for as long as you want, depending on your weight loss goal. Once reached, you’re advised to have 1 meal-replacement shake a day, up to 2 low-fat snacks, and 2 healthy meals. The plan is designed to help you lose about 1 to 2lb a week.
Pros
Meal-replacement diets can be effective at helping some people lose weight and keep it off. The plan is convenient, as the products take the guesswork out of portion control and calorie counting.
No foods are forbidden, although you’re encouraged to eat lean protein, fruit and vegetables.
Cons
On their own, meal-replacement diets do little to educate people about their eating habits and change their behaviour. There’s a risk of putting the weight back on again once you stop using the products.
You may find it hard to get your 5 A Day of fruit and veg without careful planning.
Dieticians verdict
If you don’t like the taste of the meal-replacement products, you won’t stay with the plan.
The SlimFast plan can be useful to kickstart your weight loss regime, but it’s important that you make full use of the online support to learn about the principles of healthy eating and how to manage everyday food and drink.
LighterLife diet
The LighterLife weight loss plans combine a very low-calorie diet (VLCD) with weekly counselling. With LighterLife Total, for people with a BMI of 30 or more, you eat 4 meal-replacement food packs a day – consisting of shakes, soups, mousses or bars – and no conventional food. LighterLife Lite, for those with a BMI of 25 to 30, involves eating 3 food packs a day, plus 1 meal from a list of approved foods. There is a new LighterLife Fast Plan based on the 5:2 intermittent fasting plan.
The meal plans can lead to very rapid weight loss, and you’re advised to see your GP before starting. How long you stay on the diet depends on how much weight you have to lose.
Pros
The counselling can help you understand your relationship with food, so hopefully you can make lasting changes to keep the weight off for good.
With the meal replacements, there’s no weighing or measuring, so it’s a hassle-free approach to weight loss. Having a break from real food may kick start your weight loss, and the initial rapid weight loss can be motivating.
Cons
Initial side effects of the diet can include bad breath, a dry mouth, tiredness, dizziness, insomnia, nausea and constipation from cutting down on carbs and fibre.
Surviving on a strict diet of shakes, soups and other meal replacements isn’t much fun and can feel socially isolating.
Dieticians verdict
Rapid weight loss can be motivating, but it’s unsustainable. People often regain weight after the diet and, overall, research suggests there is little difference between a VLCD and conventional weight loss after 1 to 2 years.
LighterLife’s VLCD and its counselling component may work for some – particularly people who have struggled to lose weight for years, have health problems as a result of their weight and are clinically obese with a BMI of more than 30.
A VLCD that involves eating 1,000 calories or fewer should not be followed for more than 12 continuous weeks. If you’re eating fewer than 600 calories a day, you should have medical supervision.
WeightWatchers diet
The WeightWatchers Flex programme is based on the SmartPoints system, which gives a value to foods and drink based on protein, carbs, fat and fibre content. It’s essentially a calorie-controlled diet where you get a personal daily SmartPoints allowance, which you can use how you like. There’s no limit to the amount of fruit and most veg you can eat as part of a list of zero-points foods.
There is a focus on keeping active and choosing exercise that you enjoy as a means of earning points, and there are plenty of recipes to help with the healthy eating weight loss plan. The weekly meetings and confidential weigh-ins provide support and extra motivation to encourage long-term behaviour change. The plan is designed to help you lose up to 2lb a week.
Pros
No foods are banned, so you can eat and drink what you want provided you stick to your points allowance.
The SmartPoints system is flexible, easier to follow for some than calorie counting and less restrictive than other plans. There is also online support and mobile apps, with barcode scanners to help with shopping.
Cons
When you begin, working out the points system can be just as time-consuming as simply counting calories. Some people may feel pressured into purchasing WeightWatchers-branded foods.
Some of the zero-points foods are low in fat and good sources of protein, and can be quite filling. However, although some may be difficult to eat in large quantities (such as lean chicken or eggs), these foods will still contribute to overall calorie intake so should probably not be completely unlimited.
Dieticians verdict
WeightWatchers Flex is generally well balanced and can be a foundation for long-term changes in dietary habits. The support-group approach can help keep people motivated and educate them about healthy eating.
There is a focus on including exercise as part of the plan, which can help ensure weight loss success. It’s important to appreciate the connection between the points system and calories in order to aid long-term weight management.
Rosemary Conley diet
Rosemary Conley’s diet and fitness plans combine a low-fat, low-GI diet with regular exercise. You can follow her recipes or her various diets and fitness programmes.
You’re encouraged to eat food with 5%-or-less fat, with the exception of oily fish, porridge oats and lean meat. Her online weight loss club has a range of tools and videos covering cooking classes; medical, psychological and nutritional advice; and exercises for all fitness levels. There’s also support and motivation from trained coaches.
You learn about calorie counting and portion size, which can help you sustain your weight loss beyond the programme. The diets are designed to help you lose 14lb in 7 weeks and encourage lifestyle change. How long you stay on the plan depends on your weight loss goal.
Pros
The programme is based around calories, with a focus on cutting fat. The “portion pots” – used to measure foods such as rice, cereal, pasta and baked beans – teach you about portion control.
Physical activity is an integral part of the plan, with exercise videos suitable for all ages, sizes and abilities offered online.
Cons
Some low-fat products aren’t necessarily healthier, as they can still be high in sugar and calories.
It’s unrealistic to expect people to go out with their portion pots, which means portion control may be tricky away from the home.
Dieticians verdict
The diet and exercise plans offer a balanced approach to weight loss that teaches you about portion size, the importance of regular exercise and making healthier choices. The educational element is very useful for long-term weight management once you’ve left the programme.
Sugar-free diet
As its name suggests, a sugar-free diet plan involves avoiding most, if not all, types of sugar.
Plans usually require you to cut out food and drink high in free sugars, such as fizzy drinks, breakfast cereals, flavoured yoghurts and biscuits. Some plans involve eliminating carbohydrate in all its forms – free sugar, starchy foods and fibre – but these play an important role in a healthy diet.
Pros
Cutting down on free sugars (the sugar added in foods) is a good idea because, as a nation, we consume too much sugar overall. Getting used to understanding the sugar in foods and checking the labels can be helpful.
Cons
Going completely sugar-free can be almost impossible, as that would also mean cutting out the sugar in milk and milk products, fruit and vegetables, which would not be a balanced approach.
Dieticians verdict
Cutting down on sugar in things like sugary drinks, biscuit and cakes is a good idea, but removing all sugar, including sugar in milk, fruit and vegetables, is not a sensible approach. The sugar in these foods is slowly absorbed, and these foods contain important nutrients.
Beware of some of the alternative sugar products recommended in some sugar-free plans, such as palm sugar, coconut sugar, agave and honey: these are still all sugars.
Avoid fad diets
Avoid fad diets that recommend unsafe practices, such as fasting (going without food for long periods of time) or cutting out entire food groups. These types of diets don’t work, can make you feel ill, and aren’t sustainable because they don’t teach you long-term healthy eating habits.
This isn’t to say that all commercial diet programmes are unsafe. Many are based on sound medical and scientific principles and can work well for some people.
A responsible diet programme should:
- educate you about issues such as portion size, making behavioural changes and healthy eating
- not be overly restrictive in terms of the type of foods you can eat
- be based on achieving gradual, sustainable weight loss rather than short-term rapid weight loss, which is unlikely to last
Medication
Losing weight requires a healthy diet and regular exercise. But in certain situations, prescription weight-loss medication may help.
Many different types of anti-obesity medicines have been tested in clinical trials, but only one has proved to be safe and effective: orlistat.
You can only use orlistat if a doctor or pharmacist thinks it’s the right medicine for you. In most cases, orlistat is only available on prescription. Only one product (Alli) is available over the counter directly from pharmacies, under the supervision of a pharmacist.
Orlistat works by preventing around a third of the fat from the food you eat being absorbed. The undigested fat isn’t absorbed into your body and is passed out with your faeces (stools). This will help you avoid gaining weight, but won’t necessarily cause you to lose weight.
A balanced diet and exercise programme should be started before beginning treatment with orlistat, and you should continue this programme during treatment and after you stop taking orlistat. If you don’t make these other changes in your life, medication is unlikely to work.
When orlistat should be used
Orlistat will usually only be recommended if you’ve made a significant effort to lose weight through diet, exercise or changing your lifestyle.
Even then, orlistat is only prescribed if you have a:
- body mass index (BMI) of 28 or more, and other weight-related conditions, such as high blood pressure or type 2 diabetes
- BMI of 30 or more
Before prescribing orlistat, your doctor will discuss the benefits and potential limitations with you, including any potential side effects (see below).
Treatment with orlistat must be combined with a balanced low-fat diet and other weight loss strategies, such as doing more exercise. It’s important that the diet is nutritionally balanced over three main meals.
If you’re prescribed orlistat, you’ll also be offered advice and support about diet, exercise and making lifestyle changes.
Orlistat isn’t usually recommended for pregnant or breastfeeding women.
Dosage and duration of treatment
One orlistat capsule is taken with water immediately before, during or up to one hour after, each main meal (up to a maximum of three capsules a day).
If you miss a meal, or the meal doesn’t contain any fat, you shouldn’t take the orlistat capsule. Your doctor should explain this to you, or you can check the patient information leaflet that comes with your medicine.
Treatment with orlistat should only continue beyond three months if you’ve lost 5% of your body weight. It usually starts to affect how you digest fat within one to two days.
If you haven’t lost weight after taking orlistat for three months, it’s unlikely to be an effective treatment for you. Consult your doctor or pharmacist, as it may be necessary to stop your treatment.
Taking orlistat with other health conditions
See your doctor before starting treatment with orlistat if you have another serious health condition, such as type 2 diabetes, high blood pressure, or kidney disease, which you’re taking medication for. It may be necessary to change the dose of your medicine.
If you have type 2 diabetes, it may take you longer to lose weight using orlistat, so your target weight loss after three months may therefore be slightly lower.
You’ll have a review after you’ve been using orlistat for three months. If you’ve lost weight, your doctor may suggest continuing to use orlistat for 12 months or more. They’ll discuss the benefits, limitations and side effects with you.
Side effects of orlistat
Common side effects of orlistat include:
- fatty or oily stools
- needing the toilet urgently
- passing stools more frequently
- an oily discharge from your rectum (you may have oily spots on your underwear)
- flatulence (wind)
- stomach pain
- headaches
- upper respiratory tract infections, such as a cold
These side effects are much less likely to occur if you stick to a low-fat diet.
Women taking the oral contraceptive pill should use an additional method of contraception, such as a condom, if they experience severe diarrhoea while taking orlistat. This is because the contraceptive pill may not be absorbed by your body if you have diarrhoea, so it may not be effective.
Surgery
Weight loss surgery, also called bariatric surgery, is sometimes used to treat people who are severely obese.
Bariatric surgery is usually only available to treat people with severe obesity who fulfil all of the following criteria:
- they have a BMI of 40 or more, or between 35 and 40 and another serious health condition that could be improved with weight loss, such as type 2 diabetes or high blood pressure
- all appropriate non-surgical measures have been tried, but the person hasn’t achieved or maintained adequate, clinically beneficial weight loss
- the person is fit enough to have anaesthesia and surgery
- the person has been receiving, or will receive, intensive management as part of their treatment
- the person commits to the need for long-term follow-up
Bariatric surgery may also be considered as a possible treatment option for people with a BMI of 30 to 35 who have recently (in the last 10 years) been diagnosed with type 2 diabetes.
In rare cases, surgery may be recommended as the first treatment (instead of lifestyle treatments and medication) if a person’s BMI is 50 or above.
Common weight-loss surgeries include:
- Gastric bypass surgery. In gastric bypass (Roux-en-Y gastric bypass), the surgeon creates a small pouch at the top of your stomach. The small intestine is then cut a short distance below the main stomach and connected to the new pouch. Food and liquid flow directly from the pouch into this part of the intestine, bypassing most of your stomach.
- Laparoscopic adjustable gastric banding. In this procedure, your stomach is separated into two pouches with an inflatable band. Pulling the band tight, like a belt, the surgeon creates a tiny channel between the two pouches. The band keeps the opening from expanding and is generally designed to stay in place permanently.
- Biliopancreatic diversion with duodenal switch. This procedure begins with the surgeon removing a large part of the stomach. The surgeon leaves the valve that releases food to the small intestine and the first part of the small intestine (duodenum). Then the surgeon closes off the middle section of the intestine and attaches the last part directly to the duodenum. The separated section of the intestine is reattached to the end of the intestine to allow bile and digestive juices to flow into this part of the intestine.
- Gastric sleeve. In this procedure, part of the stomach is removed, creating a smaller reservoir for food. It’s a less complicated surgery than gastric bypass or biliopancreatic diversion with duodenal switch.
Weight-loss surgery doesn’t guarantee that you’ll lose all of your excess weight or that you’ll keep it off long term. Weight-loss success after surgery depends on your commitment to making lifelong changes in your eating and exercise habits.
Treating obesity in children
Treating obesity in children usually involves improvements to diet and increasing physical activity using behaviour change strategies.
The amount of calories your child should eat each day will depend on their age and height. Your GP should be able to advise you about a recommended daily limit, and they may also be able to refer you to your local family healthy lifestyle programme.
Children over the age of five should ideally get at least one hour (60 minutes) of vigorous-intensity exercise a day, such as running or playing football or netball. Sedentary activities, such as watching television and playing computer games, should be restricted.
Read more about the physical activity guidelines for children and young people.
Referral to a specialist in treating childhood obesity may be recommended if your child develops an obesity-related complication, or there’s thought to be an underlying medical condition causing obesity.
The use of orlistat in children is only recommended in exceptional circumstances, such as if a child is severely obese and has an obesity-related complication.
Bariatric surgery isn’t generally recommended for children, but may be considered for young people in exceptional circumstances, and if they’ve achieved, or nearly achieved, physiological maturity.
New Insights Into Weight Loss Maintenance
A long-term follow-up study of participants in a televised weight loss competition suggests that there is a persistent change in how the body handles calories that can interfere with efforts to maintain weight loss. Scientists in the National Institute of Diabetes and Digestive and Kidney Diseases Intramural Research
Program 248 originally studied metabolic changes in 16 extremely obese men and women who lost weight through intensive diet and exercise in the televised “The Biggest Loser” competition. They have now conducted a follow up study with 14 of these people 6 years after the end of the 30-week competition. A key finding in the original study was that, between the start and end of the competition, participants’ weight loss was accompanied by greater than predicted and substantial reduction in their resting basal metabolic rate (BMR)—a measure of the minimum amount of calories the body will burn per day. Lowering resting basal metabolic rate is a way for the body to resist weight loss and to be able to function on fewer calories, and is advantageous in circumstances such as starvation. However, in the context of losing excess fat weight, this metabolic adaptation may contribute to weight regain, especially if it persists.
In the new study 249, the National Institute of Diabetes and Digestive and Kidney Diseases scientists obtained data on body composition so that—using an equation developed in the original study that also takes into account factors such as age and gender—they could calculate a new predicted resting BMR for each person. They also measured each person’s actual resting BMR. When they compared the data from the beginning of the competition, the end of the competition, and 6 years later, they found that all but one participant had regained at least some of their lost weight. At the same time, participants’ resting BMRs had, on average, remained at the same reduced level seen at the end of the competition, rather than increasing as would be predicted with weight regain. This alarming result suggests that metabolic adaptation following diet- and exercise-induced weight loss persists and does not fully reverse even as weight is regained, adding to people’s struggle to maintain weight loss. Encouragingly, however, when examining individual results, the researchers found that the persons who maintained greater weight loss at the 6-year mark also experienced greater ongoing metabolic slowing—suggesting that the observed BMR adaptation does not completely counter weight loss. Maintaining a lower body weight nonetheless requires continued attention to physical activity and dietary changes, given the body’s tendency to burn fewer calories after weight loss.
An additional study, published in The New England Journal of Medicine in 2010 250, looked at the role of protein and glycemic index upon weight loss maintenance. Researchers first implemented a very low-calorie diet (800 kcal per day with the use of Modifast products (Nutrition et Santé). Participants could also eat up to 400 g of vegetables, providing a total, including the very low-calorie diet, of 800 to 1000 kcal per day) to produce the weight loss, then examined whether protein and glycemic index impacted weight loss maintenance.
The study population was made up of 773 overweight adults from European countries who had lost at least 8% of their initial body weight with a very low-calorie diet. Participants were then assigned one of five diets to prevent weight regain over a 26-week period:
- Low-Protein and Low-Glycemic-Index diet,
- Low-Protein and High-Glycemic-index diet,
- High-Protein and Low-Glycemic-index diet,
- High-Protein and High-glycemic-index diet,
- or a control diet.
The low-protein-high-glycemic-index diet was associated with subsequent significant weight regain, and weight regain was less in the groups assigned to a high-protein diet than in those assigned to a low-protein diet, as well as less in the groups assigned to a low-glycemic-index diet than in those assigned to a high-glycemic-index diet.
These results show that a modest increase in protein content and a modest reduction in the glycemic index led to an improvement in maintenance of weight loss 250.
Effects of reducing or reversing the obesity epidemic
You should aim to achieve and maintain a healthy body weight.
A 1% BMI reduction (1% reduction is equivalent to a weight loss of roughly 1 kg for an adult of average weight) across the US population would avoid up to:
- 2·1–2·4 million incident cases of diabetes,
- 1·4–1·7 million cardiovascular diseases, and
- 73 000–127 000 cases of cancer, with a gain of about 16 million quality-adjusted life-years.
The equivalent scenario in the UK would avoid 179 000–202 000 incident cases of diabetes, 122 000 cardiovascular diseases, and 32 000–33 000 incident cases of cancer with a gain of about 3 million quality-adjusted life-years over 20 years.
Because 1% reduction in BMI is roughly 1 kg weight reduction per person, it would need a net caloric reduction of 20 kcal per day that was sustained for 3 years.
kcal is sometimes call kilogram calorie, it’s a measurement unit of energy. It is defined as the amount of energy needed to raise the temperature of one kilogram of water by one degree Celsius. The symbol of kilocalorie is kcal. The kilocalorie is commonly used in measuring the calorific, heating, or metabolizing value of foods. It is equal to 1000 calories, or approximately 4.2 kilojoules.
New Directions in Obesity Treatments
Brain Stimulation with Electric Current Leads to Changes in Food Consumption and Body Weight in a Preliminary Study of Adults with Obesity
- In an intriguing preliminary study, researchers found that a method for stimulating brain activity with electricity, transcranial direct current stimulation (tDCS) – non-invasive but still experimental method, affected food choices of people with obesity and led to a small amount of weight loss over several days. For the next 3 mornings, subjects were randomly assigned to receive either tDCS or the sham treatment. After their treatments, they ate all of their food from special computerized vending machines; they could choose whatever they wanted to eat and drink from the vending machines, and could eat whenever and as much as they wished. Their food choices, the amounts they consumed, and their body weights were recorded. Five of the volunteers received an inactive form of tDCS on their first visit to the research center, and active tDCS on the second visit. These individuals consumed significantly fewer calories from fat and soda and lost more weight during the visit in which they received the active tDCS. The four volunteers who received the sham treatment on both visits to the center did not experience these changes 251.
- Vagal nerve blockade is another treatment for obesity. It involves implanting a device under the skin of the abdomen that sends intermittent electrical pulses to the abdominal vagus nerve, which tells the brain when the stomach feels empty or full. This new technology received FDA approval in 2014 for use by adults who have not been able to lose weight with a weight-loss program and who have a BMI of 35 to 45 with at least one obesity-related condition, such as type 2 diabetes.
Naturally Occurring Compound Shows Potential as a Treatment for Obesity and Diabetes
- Researchers have used an innovative drug-discovery approach to identify a naturally occurring compound, called withaferin A, that mitigates obesity and its metabolic effects in mice. Fat cells secrete a hormone called leptin, which signals to the body to stop eating when energy stores are sufficient. When leptin was first discovered in the 1990’s, it was thought that the hormone may be useful to treat obesity. However, except in rare cases of obesity caused by leptin deficiency, obese individuals actually have high levels of leptin, but they are resistant to leptin’s actions. Thus, in obese individuals who are leptin resistant,
the hormone is unable to curb appetite, resulting in overeating and additional weight gain. Through previous research they identified a naturally occurring compound, called celastrol, which increased leptin sensitivity and promoted weight loss in mice. They looked for compounds that induced a similar gene expression profile—i.e., the genes that are turned on and off—in cells as when they are treated with celastrol. They reasoned that such compounds may similarly improve leptin sensitivity. This approach led to the discovery of withaferin A. Withaferin-A treatment of mice that were obese and leptin-resistant because of eating a high-fat diet led to a 23 percent reduction in body weight and a 35 percent reduction in fat mass compared to control mice; treated animals also ate substantially less food. Withaferin-A treatment also resolved the animals’ fatty liver disease, a condition associated with obesity. In contrast, withaferin A did not reduce the body weight of lean mice, which are not leptin resistant, and only marginally affected the body weight of mice that do not make leptin because of a genetic mutation, and thus would not be expected to be affected by a therapy that improves leptin sensitivity 252.
Custom-made Fat Tissue That Burns Calories
In research 253 that might lead to a new obesity and diabetes treatment approach, scientists developed a novel technique in mice for directing stem cells from body fat to grow in special gels and form fat tissue that burns—rather than stores—calories. Based on earlier findings that some types of body fat, called brown and beige fat, can generate heat by burning stored calories, researchers have proposed various strategies for creating more of these types of tissues to reduce excess weight and boost metabolism. Pursuing one such strategy, a multidisciplinary research team sought to grow beige fat tissue in the lab and test whether it would improve weight and health in mice.
- Swinburn, BA, Sacks, G, Hall, KD et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011; 377: 804–814. http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(11)60813-1/fulltext[↩][↩]
- Cutler, DM, Glaeser, EL, and Shapiro, JM. Why have Americans become more obese?. J Econ Perspect. 2003; 17: 93–118.[↩][↩][↩][↩]
- McCrory, MA, Suen, VM, and Roberts, SB. Biobehavioral influences on energy intake and adult weight gain. J Nutr. 2002; 132: 3830S–3834S. http://jn.nutrition.org/content/132/12/3830S.long[↩]
- Prentice, A and Jebb, S. Energy intake/physical activity interactions in the homeostasis of body weight regulation. Nutr Rev. 2004; 62: S98–104. https://www.ncbi.nlm.nih.gov/pubmed/15387474[↩]
- Hall, KD, Guo, J, Dore, M, and Chow, CC. The progressive increase of food waste in America and its environmental impact. PLoS One. 2009; 4: e7940. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2775916/[↩]
- Bleich, S, Cutler, D, Murray, C, and Adams, A. Why is the developed world obese?. Annu Rev Public Health. 2008; 29: 273–295. https://www.ncbi.nlm.nih.gov/pubmed/18173389[↩]
- Goris, JM, Petersen, S, Stamatakis, E, and Veerman, JL. Television food advertising and the prevalence of childhood overweight and obesity: a multicountry comparison. Public Health Nutr. 2010; 13: 1003–1012. https://www.ncbi.nlm.nih.gov/pubmed/20018123[↩]
- Committee on Food Marketing and the Diets of Children and Youth. Food marketing to children and youth: threat or opportunity?National Academies Press, Washington, DC; 2006.[↩]
- Cairns, G, Angus, K, and Hastings, G. The extent, nature and effects of food promotion to children: a review of the evidence to December 2008. World Health Organization, Geneva; 2009.[↩]
- Bouchard, C. Gene-environment interactions in the etiology of obesity: defining the fundamentals. Obesity (Silver Spring). 2008; 16: S5–10. https://www.ncbi.nlm.nih.gov/pubmed/19037213[↩]
- Ahmed, F. Epigenetics: tales of adversity. Nature. 2010; 468: S20. https://www.ncbi.nlm.nih.gov/pubmed/21179081[↩]
- Kessler, D. The End of Overeating: Taking Control of the Insatiable American Appetite. Rodale Books, Emaus, PA; 2009.[↩]
- Sallis, JF. Age-related decline in physical activity: a synthesis of human and animal studies. Med Sci Sports Exerc. 2000; 32: 1598–1600. https://www.ncbi.nlm.nih.gov/pubmed/10994911[↩]
- BMI Calculator Adults. https://www.cdc.gov/healthyweight/assessing/bmi/adult_BMI/english_bmi_calculator/bmi_calculator.html[↩]
- BMI Calculator Children. https://nccd.cdc.gov/dnpabmi/Calculator.aspx[↩]
- World Cancer Research Fund and American Institute for Cancer Research. food, nutrition, physical activity, and the prevention of cancer: a global perspective, 2007. American Institute for Cancer Research, Washington, DC; 2007.[↩]
- M Ezzati, A Lopez, AD Rodgers, CJL Murray (Eds.) Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors. World Health Organization, Geneva; 2004.[↩]
- Australian Institute of Health and Welfare (AIHW) and National Heart Foundation of Australia. The relationship between overweight, obesity and cardiovascular disease. AIHW (Cardiovascular Disease Series No. 23), Canberra; 2004.[↩]
- Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA 2013; 309(1):71-82. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4855514/[↩]
- Kitahara CM, Flint AJ, Berrington de Gonzalez A, et al. Association between class III obesity (BMI of 40-59 kg/m2) and mortality: a pooled analysis of 20 prospective studies. PLoS Medicine 2014; 11(7):e1001673. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4087039/[↩]
- National Cancer Institute. Obesity and Cancer. https://www.cancer.gov/about-cancer/causes-prevention/risk/obesity/obesity-fact-sheet[↩]
- National Cancer Institute. Obesity. https://www.cancer.gov/about-cancer/causes-prevention/risk/obesity[↩][↩]
- Whiteman DC, Wilson LF. The fractions of cancer attributable to modifiable factors: A global review. Cancer Epidemiology 2016; 44:203-221. https://www.ncbi.nlm.nih.gov/pubmed/27460784[↩][↩]
- Arnold M, Pandeya N, Byrnes G, et al. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncology 2015; 16(1):36-46. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4314462/[↩]
- National Institutes of Health. Overweight and Obesity. https://www.nhlbi.nih.gov/health/health-topics/topics/obe[↩]
- Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA 2013; 309(1):71-82. www.ncbi.nlm.nih.gov/pubmed/23280227[↩]
- Kitahara CM, Flint AJ, Berrington de Gonzalez A, et al. Association between class III obesity (BMI of 40-59 kg/m2) and mortality: a pooled analysis of 20 prospective studies. PLoS Medicine 2014; 11(7):e1001673. www.ncbi.nlm.nih.gov/pubmed/25003901[↩]
- National Heart, Lung and Blood Institute. BMI Calculator. https://www.nhlbi.nih.gov/health/educational/lose_wt/BMI/bmicalc.htm[↩]
- Centers for Disease Control and Prevention. CDC Clinical Growth Charts. https://www.cdc.gov/growthcharts/clinical_charts.htm[↩]
- Centers for Disease Control and Prevention. BMI Percentile Calculator for Child and Teen. https://nccd.cdc.gov/dnpabmi/Calculator.aspx[↩]
- Greeno CG, Wing RR. Stress-induced eating. Psychol Bull 1994; 115: 444–464. https://www.ncbi.nlm.nih.gov/pubmed/8016287[↩][↩]
- Adams CE, Leary MR. Promoting self-compassionate attitudes toward eating among restrictive and guilty eaters. J Soc Clin Psychol 2007; 26: 1120–1144.[↩][↩]
- Mathes WF, et al. “The Biology of Binge Eating,” Appetite (June 2009): Vol. 52, No. 3, pp. 545–53.[↩][↩]
- American Psychological Association. Stress in America Press Room. http://www.apa.org/news/press/releases/stress/index.aspx[↩][↩]
- Harvard University.Harvard Medical School. Why stress causes people to overeat. http://www.health.harvard.edu/newsletter_article/why-stress-causes-people-to-overeat[↩][↩][↩][↩]
- Spencer SJ, et al. “The Glucocorticoid Contribution to Obesity,” Stress (Feb. 6, 2011): Vol. 14, No. 3, pp. 233–46.[↩][↩]
- Vicennati V, et al. “Stress-Related Development of Obesity and Cortisol in Women,” Obesity (Sept. 2009): Vol. 17, No. 9, pp. 1678–83.[↩][↩]
- Kawakami A, Okada N, Rokkaku K, Honda K, Ishibashi S, Onaka T. Leptin inhibits and ghrelin augments hypothalamic noradrenaline release after stress. Stress 2008; 11: 363–369. https://www.ncbi.nlm.nih.gov/pubmed/18800308[↩][↩]
- Adams CE, et al. “Lifestyle Factors and Ghrelin: Critical Review and Implications for Weight Loss Maintenance,” Obesity Review (May 2011): Vol. 12, No. 5, electronic publication.[↩][↩]
- Rouach V, Bloch M, Rosenberg N, Gilad S, Limor R, Stern N, Greenman Y. The acute ghrelin response to a psychological stress challenge does not predict the post-stress urge to eat. Psychoneuroendocrinology 2007; 32: 693–702. https://www.ncbi.nlm.nih.gov/pubmed/17560728[↩][↩][↩]
- Obesity Reviews Volume 12, Issue 5, May 2011. Pages e211–e218. Lifestyle factors and ghrelin: critical review and implications for weight loss maintenance. http://onlinelibrary.wiley.com/doi/10.1111/j.1467-789X.2010.00776.x/full[↩][↩]
- Manzoni GM, et al. “Can Relaxation Training Reduce Emotional Eating in Women with Obesity?” Journal of the American Dietetic Association (Aug. 2009): Vol. 109, No. 8, pp. 1427–32.[↩]
- Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring). 2008; 16:643-53. https://www.ncbi.nlm.nih.gov/pubmed/18239586[↩][↩][↩][↩][↩][↩]
- Why Is Sleep Important ? National Heart, Lung and Blood Institute. https://www.nhlbi.nih.gov/health/health-topics/topics/sdd/why[↩][↩][↩]
- Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004; 1:e62. https://www.ncbi.nlm.nih.gov/pubmed/15602591[↩][↩]
- Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004; 141:846-50. https://www.ncbi.nlm.nih.gov/pubmed/15583226[↩][↩]
- Taheri S. The link between short sleep duration and obesity: we should recommend more sleep to prevent obesity. Arch Dis Child. 2006; 91:881-4. https://www.ncbi.nlm.nih.gov/pubmed/17056861[↩][↩]
- Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009; 89:126-33. https://www.ncbi.nlm.nih.gov/pubmed/19056602[↩][↩]
- Imaki M, Hatanaka Y, Ogawa Y, Yoshida Y, Tanada S. An epidemiological study on relationship between the hours of sleep and life style factors in Japanese factory workers. J Physiol Anthropol Appl Human Sci. 2002; 21:115-20. https://www.ncbi.nlm.nih.gov/pubmed/12056178[↩][↩]
- Patel SR, Malhotra A, White DP, Gottlieb DJ, Hu FB. Association between reduced sleep and weight gain in women. Am J Epidemiol. 2006; 164:947-54. https://www.ncbi.nlm.nih.gov/pubmed/16914506[↩][↩][↩][↩]
- Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring). 2008; 16:643-53. www.ncbi.nlm.nih.gov/pubmed/18239586[↩][↩][↩][↩]
- Manini TM, Everhart JE, Patel KV, et al. Daily activity energy expenditure and mortality among older adults. JAMA. 2006; 296:171-9. https://www.ncbi.nlm.nih.gov/pubmed/16835422[↩][↩]
- Twelve Simple Tips to Improve Your Sleep. 2007. http://healthysleep.med.harvard.edu/healthy/getting/overcoming/tips[↩]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of Obesity in the United States, 2009–2010. NCHS Data Brief. 2012;Jan(82):1-8.[↩][↩]
- Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307(5):491-7. [↩]
- National Center for Health Statistics. Health, United States, 2015: With Special Feature on Racial and Ethnic Health Disparities. Hyattsville, MD. 2016. https://www.cdc.gov/nchs/data/hus/hus15.pdf[↩][↩]
- Ogden CL, Carroll MD, Lawman HG, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988-1994 through 2013-2014. JAMA 2016; 315(21):2292-2299. https://www.ncbi.nlm.nih.gov/pubmed/27272581[↩]
- Ogden CL, Carroll MD. Prevalence of Overweight, Obesity, and Extreme Obesity Among Adults: United States, Trends 1960–1962 Through 2007–2008. Hyattsville, MD: National Center for Health Statistics, 2010.[↩]
- Centers for Disease Control and Prevention. Adult Obesity Prevalence Maps. https://www.cdc.gov/obesity/data/prevalence-maps.html[↩]
- CDC/NCHS. National Health and Nutrition Examination Survey 1988–194, 1999–2000, 2001–2002, 2003–2004, 2005–2006, 2007–2008, and 2009–2010.[↩]
- Flegal et al. JAMA 315: 2284-2291, 2016.[↩]
- Ogden et al. JAMA 315: 2292-2299, 2016.[↩]
- Finucane, MM, Stevens, GA, Cowan, MJ et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet. 2011; 377: 557–567. http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(10)62037-5/fulltext[↩]
- Butland, B, Jebb, S, Kopelma, P et al. Tackling obesities: future choices—project report. Government Office for Science, London; 2007.[↩]
- U.S. Department of Health and Human Services, National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/about-niddk/strategic-plans-reports/Documents/2017_NIDDK_RecentAdvancesEmergingOpps_Obesity.pdf[↩][↩]
- Li Y-Q, Shrestha Y, Pandey M,…Weinstein LS. Gq/11α and Gsα mediate distinct physiological responses to central melanocortins. J Clin Invest 126: 40-49, 2016.[↩][↩]
- Nakajima K, Cui Z, Li C,…Wess J. Gs-coupled GPCR signaling in AgRP neurons triggers sustained increase in food intake. Nat Communications 7: 10268, 2016.[↩]
- Lagerlöf O, Slocomb JE, Hong I,…Huganir RL. The nutrient sensor OGT in PVN neurons regulates feeding. Science 351: 1293-1296, 2016.[↩]
- Williams EK, Chang RB, Strochlic DE, Umans BD, Lowell BB, and Liberles SD. Sensory neurons that detect stretch and nutrients in the digestive system. Cell 166: 209-221, 2016.[↩]
- Perry RJ, Peng L, Barry NA, Shulman GI. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature 534: 213-217, 2016.[↩][↩]
- Siiteri PK. 1987. Adipose tissue as a source of hormones. Am J Clin Nutr 45:277–282. https://www.ncbi.nlm.nih.gov/pubmed/3541569[↩]
- Flier JS, Cook KS, Usher P, Spiegelman BM. Science. 1987 Jul 24;237(4813):405-8. Severely impaired adipsin expression in genetic and acquired obesity. https://www.ncbi.nlm.nih.gov/pubmed/3299706[↩]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Nature. 1994 Dec 1;372(6505):425-32. Positional cloning of the mouse obese gene and its human homologue. https://www.ncbi.nlm.nih.gov/pubmed/7984236[↩]
- Erin E. Kershaw, Jeffrey S. Flier; Adipose Tissue as an Endocrine Organ, The Journal of Clinical Endocrinology & Metabolism, Volume 89, Issue 6, 1 June 2004, Pages 2548–2556, https://doi.org/10.1210/jc.2004-0395. https://academic.oup.com/jcem/article-lookup/doi/10.1210/jc.2004-0395[↩][↩]
- Circulation. 2004 Jan 27;109(3):433-8. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. http://circ.ahajournals.org/content/109/3/433.long[↩][↩]
- Leow MK, Addy CL, Mantzoros CS. J Clin Endocrinol Metab. 2003 May;88(5):1961-76. Clinical review 159: Human immunodeficiency virus/highly active antiretroviral therapy-associated metabolic syndrome: clinical presentation, pathophysiology, and therapeutic strategies. https://www.ncbi.nlm.nih.gov/pubmed/12727939[↩][↩]
- Ahima RS, Flier JS. Trends Endocrinol Metab. 2000 Oct;11(8):327-32. Adipose tissue as an endocrine organ. https://www.ncbi.nlm.nih.gov/pubmed/10996528[↩]
- Fruhbeck G, Gomez-Ambrosi J, Muruzabal FJ, Burrell MA 2001 The adipocyte: a model for integration of endocrine and metabolic signaling in energy metabolism regulation. Am J Physiol Endocrinol Metab 280:E827—E847.[↩]
- Frayn KN, Karpe F, Fielding BA, Macdonald IA, Coppack SW. Int J Obes Relat Metab Disord. 2003 Aug;27(8):875-88. Integrative physiology of human adipose tissue. https://www.ncbi.nlm.nih.gov/pubmed/12861227[↩][↩]
- Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Endocrinology. 2004 May;145(5):2273-82. Epub 2004 Jan 15. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. https://www.ncbi.nlm.nih.gov/pubmed/14726444[↩]
- Friedman JM, Halaas JL. Nature. 1998 Oct 22;395(6704):763-70. Leptin and the regulation of body weight in mammals. https://www.ncbi.nlm.nih.gov/pubmed/9796811[↩]
- Bjørbaek C, Kahn BB. Recent Prog Horm Res. 2004;59:305-31. Leptin signaling in the central nervous system and the periphery. https://www.ncbi.nlm.nih.gov/pubmed/14749508[↩]
- Flier JSJ. Clin Endocrinol Metab. 1998 May;83(5):1407-13.Clinical review 94: What’s in a name? In search of leptin’s physiologic role. https://www.ncbi.nlm.nih.gov/pubmed/9589630[↩]
- Recent Prog Horm Res. 2004;59:305-31. Leptin signaling in the central nervous system and the periphery. https://www.ncbi.nlm.nih.gov/pubmed/14749508[↩][↩][↩]
- Cell. 2004 Jan 23;116(2):337-50. Obesity wars: molecular progress confronts an expanding epidemic. https://www.ncbi.nlm.nih.gov/pubmed/14744442[↩][↩]
- Flier JS, Harris M, Hollenberg AN. Leptin, nutrition, and the thyroid: the why, the wherefore, and the wiring. Journal of Clinical Investigation. 2000;105(7):859-861. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC377492/[↩]
- Stanley M. Hileman, Dominique D. Pierroz, Jeffrey S. Flier; Leptin, Nutrition, and Reproduction: Timing Is Everything, The Journal of Clinical Endocrinology & Metabolism, Volume 85, Issue 2, 1 February 2000, Pages 804–807, https://doi.org/10.1210/jcem.85.2.6490. https://academic.oup.com/jcem/article-lookup/doi/10.1210/jcem.85.2.6490[↩]
- Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. Journal of Clinical Investigation. 2003;111(9):1409-1421. doi:10.1172/JCI200317490. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC154448/[↩]
- Int J Obes Relat Metab Disord. 2002 Nov;26(11):1407-33. Leptin: a review of its peripheral actions and interactions. https://www.ncbi.nlm.nih.gov/pubmed/12439643[↩][↩]
- Nature. 1998 Aug 27;394(6696):897-901. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. https://www.ncbi.nlm.nih.gov/pubmed/9732873[↩]
- Lancet. 2003 Nov 8;362(9395):1572-4. Leptin: cutting the fat off the bone. https://www.ncbi.nlm.nih.gov/pubmed/14615115[↩][↩][↩]
- Cytokine Growth Factor Rev. 2003 Oct;14(5):447-55. Insulin resistance in adipose tissue: direct and indirect effects of tumor necrosis factor-alpha. https://www.ncbi.nlm.nih.gov/pubmed/12948526[↩][↩][↩][↩][↩]
- Endocrinology. 2004 May;145(5):2273-82. Epub 2004 Jan 15. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. https://www.ncbi.nlm.nih.gov/pubmed/14726444[↩][↩][↩][↩]
- Endocr Rev. 2000 Dec;21(6):697-738. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. https://www.ncbi.nlm.nih.gov/pubmed/11133069[↩]
- Int J Obes Relat Metab Disord. 2003 Dec;27 Suppl 3:S53-5. Inflammatory pathways and insulin action. https://www.ncbi.nlm.nih.gov/pubmed/14704746[↩][↩]
- Science. 1993 Jan 1;259(5091):87-91. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. https://www.ncbi.nlm.nih.gov/pubmed/7678183[↩][↩][↩][↩][↩]
- Nature. 1997 Oct 9;389(6651):610-4. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. https://www.ncbi.nlm.nih.gov/pubmed/9335502[↩]
- Diabetes. 2002 Nov;51(11):3176-88. Profiling gene transcription in vivo reveals adipose tissue as an immediate target of tumor necrosis factor-alpha: implications for insulin resistance. https://www.ncbi.nlm.nih.gov/pubmed/12401708[↩][↩][↩]
- J Biol Chem. 1996 May 3;271(18):10697-703. AdipoQ is a novel adipose-specific gene dysregulated in obesity. https://www.ncbi.nlm.nih.gov/pubmed/8631877[↩]
- Diabetes Care. 2003 Aug;26(8):2442-50. Adiponectin: more than just another fat cell hormone ? https://www.ncbi.nlm.nih.gov/pubmed/12882876[↩][↩][↩][↩]
- Eur J Endocrinol. 2003 Mar;148(3):293-300. The role of the novel adipocyte-derived hormone adiponectin in human disease. https://www.ncbi.nlm.nih.gov/pubmed/12611609[↩]
- Diabetes. 2001 May;50(5):1126-33. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. https://www.ncbi.nlm.nih.gov/pubmed/11334417[↩]
- Endocrinology. 2004 Feb;145(2):484-6. Adiponectin and HIV-lipodystrophy: taking HAART. https://www.ncbi.nlm.nih.gov/pubmed/14739152[↩]
- Nat Med. 2002 Jul;8(7):731-7. Epub 2002 Jun 17. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. https://www.ncbi.nlm.nih.gov/pubmed/12068289[↩][↩][↩][↩]
- J Clin Invest. 2003 Dec;112(12):1785-8. Obesity-induced inflammatory changes in adipose tissue. https://www.ncbi.nlm.nih.gov/pubmed/14679172[↩][↩][↩]
- J Biol Chem. 2003 Nov 21;278(47):46654-60. Epub 2003 Sep 16. Adiposity elevates plasma MCP-1 levels leading to the increased CD11b-positive monocytes in mice. https://www.ncbi.nlm.nih.gov/pubmed/13129912[↩]
- Proc Natl Acad Sci U S A. 2003 Jun 10;100(12):7265-70. Epub 2003 May 19. Monocyte chemoattractant protein 1 in obesity and insulin resistance. https://www.ncbi.nlm.nih.gov/pubmed/12756299[↩]
- Endocr Rev. 2003 Jun;24(3):278-301. Insulin resistance and chronic cardiovascular inflammatory syndrome. https://www.ncbi.nlm.nih.gov/pubmed/12788800[↩][↩][↩][↩][↩][↩][↩][↩]
- J Biol Chem. 2003 Apr 18;278(16):13740-6. Epub 2003 Jan 30. Suppressor of cytokine signaling-3 (SOCS-3), a potential mediator of interleukin-6-dependent insulin resistance in hepatocytes. https://www.ncbi.nlm.nih.gov/pubmed/12560330[↩]
- Obes Rev. 2002 May;3(2):85-101. Obesity, haemostasis and the fibrinolytic system. https://www.ncbi.nlm.nih.gov/pubmed/12120424[↩][↩][↩][↩]
- J Thromb Haemost. 2003 Jul;1(7):1575-9. Plasminogen activator inhibitor-1, inflammation, obesity, insulin resistance and vascular risk. https://www.ncbi.nlm.nih.gov/pubmed/12871293[↩][↩]
- Biochim Biophys Acta. 2003 Jan 31;1609(2):127-43. Critical review of acylation-stimulating protein physiology in humans and rodents. https://www.ncbi.nlm.nih.gov/pubmed/12543373[↩][↩][↩][↩][↩][↩]
- Science. 1987 Jul 24;237(4813):405-8. Severely impaired adipsin expression in genetic and acquired obesity. https://www.ncbi.nlm.nih.gov/pubmed/3299706[↩]
- J Biol Chem. 2004 Feb 6;279(6):4051-7. Epub 2003 Nov 13. Acylation-stimulating protein (ASP)/complement C3adesArg deficiency results in increased energy expenditure in mice. https://www.ncbi.nlm.nih.gov/pubmed/14615480[↩]
- J Biol Chem. 2003 Mar 28;278(13):11123-9. Epub 2003 Jan 22. The chemoattractant receptor-like protein C5L2 binds the C3a des-Arg77/acylation-stimulating protein. https://www.ncbi.nlm.nih.gov/pubmed/12540846[↩]
- Arnold M, Pandeya N, Byrnes G, et al. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncology 2015; 16(1):36-46. www.ncbi.nlm.nih.gov/pubmed/25467404[↩]
- National Institutes of Health, National Heart, Lung and Blood Institute. Causes of Overweight and Obesity. https://www.nhlbi.nih.gov/health/health-topics/topics/obe/causes[↩]
- Kitchen, P, Brignell, J, Li, T, and Spickett-Jones, G. The emergence of IMC: a theoretical perspective. J Advertising Res. 2004; March: 19–30.[↩]
- Hall, KD, Guo, J, Dore, M, and Chow, CC. The progressive increase of food waste in America and its environmental impact. PLoS ONE. 2009; 4: e7940. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2775916/[↩][↩][↩]
- Swinburn, B, Sacks, G, and Ravussin, E. Increased food energy supply is more than sufficient to explain the US epidemic of obesity. Am J Clin Nutr. 2009; 90: 1453–1456. http://ajcn.nutrition.org/content/90/6/1453.long[↩][↩]
- Scarborough, P, Burg, MR, Foster, C et al. Increased energy intake entirely accounts for increase in body weight in women but not in men in the UK between 1986 and 2000. Br J Nutr. 2011; 105: 1399–1404. https://www.ncbi.nlm.nih.gov/pubmed/21205425[↩][↩]
- Economic Research Service, US Department of Agriculture. Food availability (per capita) data system. Washington, DC: US Department of Agriculture. https://www.ers.usda.gov/Data/FoodConsumption[↩][↩]
- Gerrior, S, Bente, L, and Hiza, H. Nutrient content of the U.S. Food Supply. 1909–2000. US Department of Agriculture, Center for Nutrition Policy and Promotion, Washington, DC; 2004.[↩]
- Putnam, J. Major trends in the U.S. food supply, 1909–99. Food Rev. 2000; 23: 8–15.[↩]
- Putnam, J, Allshouse, J, and Kantor, L. US per capita food supply trends: more calories, refined carbohydrates, and fats. Food Rev. 2002; 25: 2–15.[↩]
- Putnam, JKL and Allshouse, J. Per capita food supply trends: progress towards dietary guidelines. Food Rev. 2000; 23: 2–14.[↩]
- Hall, KD, Sacks, G, Chandramohan, D et al. Quantifying the effect of energy imbalance on bodyweight change. Lancet. 2011; 378: 826–837. http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(11)60812-X/fulltext[↩]
- Mattes RD. Fluid energy—Where’s the problem? J Am Diet Assoc. 2006;106(12):1956–1961. https://www.ncbi.nlm.nih.gov/pubmed/17126624[↩]
- Mattes RD, Campbell WW. Effects of food form and timing of ingestion on appetite and energy intake in lean young adults and in young adults with obesity. J Am Diet Assoc. 2009;109(3):430–437. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2680008/[↩][↩]
- Rolls BJ, Kim S, Fedoroff IC. Effects of drinks sweetened with sucrose or aspartame on hunger, third and food intake in men. Physiol Behav. 1990;48(1):19–26. https://www.ncbi.nlm.nih.gov/pubmed/2236270[↩][↩]
- Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364:2392-404. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3151731/[↩]
- Guthrie JF, Morton JF. Food sources of added sweeteners in the diets of Americans. J Am Diet Assoc 2000;100(51):43-48.[↩]
- Wang YC, Bleich SN, Gortmaker SL. Increasing caloric contribution from sugar-sweetened beverages and 100% fruit juices among US children and adolescents, 1988-2004. Pediatrics 2008;121(6):e1604-1614.[↩]
- Frazao E, Allshouse J. Strategies for intervention: commentary and debate. J of Nutr 2003;844S-847S.[↩]
- Briefel RR, Johnson CL. Secular trends in dietary intake in the United States. Annu Rev Nutr 2004;24:401-431.[↩][↩]
- Circulation. 2016;CIRCULATION AHA.115.018704. Sugar-Sweetened Beverage Consumption is Associated With Change of Visceral Adipose Tissue Over 6 Years of Follow-Up. http://circ.ahajournals.org/content/early/2016/01/06/CIRCULATIONAHA.115.018704[↩]
- Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, McGahan JP, Seibert A, Krauss RM, Chiu S, Schaefer EJ, Ai M, Otokozawa S, Nakajima K, Nakano T, Beysen C, Hellerstein MK, Berglund L, Havel PJ. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119(5):1322–1334. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2673878/[↩]
- Circulation. 2009 Sep 15;120(11):1011-20. doi: 10.1161/CIRCULATIONAHA.109.192627. Epub 2009 Aug 24. Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association. https://www.ncbi.nlm.nih.gov/pubmed/19704096/[↩]
- Block G. Foods contributing to energy intake in the US: data from NHANES III and NHANES 1999-2000. Journal of Food Composition and Analysis. 2004;14:439–447. [↩]
- Int J Obes Relat Metab Disord. 2000 Jun;24(6):794-800. Liquid versus solid carbohydrate: effects on food intake and body weight. https://www.ncbi.nlm.nih.gov/pubmed/10878689/[↩]
- Physiol Behav. 1996 Jan;59(1):179-87. Dietary compensation by humans for supplemental energy provided as ethanol or carbohydrate in fluids. https://www.ncbi.nlm.nih.gov/pubmed/8848479/[↩]
- Am J Clin Nutr. 2004 Aug;80(2):348-56. Glycemic index, glycemic load, and dietary fiber intake and incidence of type 2 diabetes in younger and middle-aged women. https://www.ncbi.nlm.nih.gov/pubmed/15277155/[↩]
- Nielsen SJ, Siega-Riz AM, Popkin BM. Trends in energy intake in U.S. between 1977 and 1996: similar shifts seen across age groups. Obes Res 2002;10(5):370-378.[↩][↩]
- French SA, Story M, Jeffery RW. Environmental influences on eating and physical activity. Ann Rev Public Health 2001;22:308-335.[↩][↩][↩]
- Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295(13):1549-1555.[↩]
- Agras WS, Mascola AJ. Risk factors for childhood overweight. Curr Opin Pediatr 2005;17(5):648-652.[↩]
- Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr 2003;77:1146-1155.[↩]
- National Institute of Diabetes and Digestive and Kidney Diseases. Body Weight Planner. https://www.supertracker.usda.gov/bwp/index.html[↩]
- Am J Clin Nutr. 2004 Apr;79(4):537-43. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. https://www.ncbi.nlm.nih.gov/pubmed/15051594/[↩][↩]
- Nutr Rev. 2005 May;63(5):133-57. Dietary fructose: implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. https://www.ncbi.nlm.nih.gov/pubmed/15971409/[↩]
- The 2015-2020 Dietary Guidelines for Americans. https://health.gov/dietaryguidelines/2015/[↩]
- Reilly JJ, Armstrong J, Dorosty AR, et al. Early life risk factors for obesity in childhood: cohort study. BMJ. 2005; 330:1357. https://www.ncbi.nlm.nih.gov/pubmed/15908441[↩][↩]
- Agras WS, Hammer LD, McNicholas F, Kraemer HC. Risk factors for childhood overweight: a prospective study from birth to 9.5 years. J Pediatr. 2004; 145:20-5. https://www.ncbi.nlm.nih.gov/pubmed/15238901[↩]
- Gillman MW, Rifas-Shiman SL, Kleinman K, Oken E, Rich-Edwards JW, Taveras EM. Developmental origins of childhood overweight: potential public health impact. Obesity (Silver Spring). 2008; 16:1651-6. https://www.ncbi.nlm.nih.gov/pubmed/18451768[↩]
- Taveras EM, Rifas-Shiman SL, Oken E, Gunderson EP, Gillman MW. Short sleep duration in infancy and risk of childhood overweight. Arch Pediatr Adolesc Med. 2008; 162:305-11. https://www.ncbi.nlm.nih.gov/pubmed/18391138[↩][↩]
- Bell JF, Zimmerman FJ. Shortened nighttime sleep duration in early life and subsequent childhood obesity. Arch Pediatr Adolesc Med. 2010; 164:840-5.[↩]
- Landhuis CE, Poulton R, Welch D, Hancox RJ. Childhood sleep time and long-term risk for obesity: a 32-year prospective birth cohort study. Pediatrics. 2008; 122:955-60. https://www.ncbi.nlm.nih.gov/pubmed/18977973[↩][↩]
- Nevarez MD, Rifas-Shiman SL, Kleinman KP, Gillman MW, Taveras EM. Associations of early life risk factors with infant sleep duration. Acad Pediatr. 2010; 10:187-93. https://www.ncbi.nlm.nih.gov/pubmed/20347414[↩]
- Wake M, Price A, Clifford S, Ukoumunne OC, Hiscock H. Does an intervention that improves infant sleep also improve overweight at age 6? Follow-up of a randomised trial. Arch Dis Child. 2011; 96:526-32. https://www.ncbi.nlm.nih.gov/pubmed/21402578[↩]
- Paul IM, Savage JS, Anzman SL, et al. Preventing obesity during infancy: a pilot study. Obesity (Silver Spring). 2011; 19:353-61. https://www.ncbi.nlm.nih.gov/pubmed/20725058[↩][↩]
- Prevention of Overweight in Infancy (POInz). ClinicalTrials.gov, 2009. https://clinicaltrials.gov/show/NCT00892983 [↩]
- Nielsen LS, Danielsen KV, Sorensen TI. Short sleep duration as a possible cause of obesity: critical analysis of the epidemiological evidence. Obes Rev. 2011; 12:78-92. https://www.ncbi.nlm.nih.gov/pubmed/20345429[↩][↩]
- Pan A, Schernhammer ES, Sun Q, Hu FB. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med. 2011; 8:e1001141. Epub 2011 Dec 6. https://www.ncbi.nlm.nih.gov/pubmed/22162955[↩]
- Chaput JP, Despres JP, Bouchard C, Tremblay A. The association between sleep duration and weight gain in adults: a 6-year prospective study from the Quebec Family Study. Sleep. 2008; 31:517-23. www.ncbi.nlm.nih.gov/pubmed/18457239[↩]
- Cizza G, Marincola P, Mattingly M, et al. Treatment of obesity with extension of sleep duration: a randomized, prospective, controlled trial. Clin Trials. 2010; 7:274-85. https://www.ncbi.nlm.nih.gov/pubmed/20423926[↩]
- Chuang J-C, Zigman JM. Ghrelin’s Roles in Stress, Mood, and Anxiety Regulation. International Journal of Peptides. 2010;2010:460549. doi:10.1155/2010/460549. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2915752/[↩][↩]
- Simon GE, Von Korff M, Saunders K, et al. Association between obesity and psychiatric disorders in the US adult population. Archives of General Psychiatry. 2006;63(7):824–830. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1913935/[↩]
- Kloiber S, Ising M, Reppermund S, et al. Overweight and obesity affect treatment response in major depression. Biological Psychiatry. 2007;62(4):321–326. https://www.ncbi.nlm.nih.gov/pubmed/17241618[↩]
- Richardson LP, Davis R, Poulton R, et al. A longitudinal evaluation of adolescent depression and adult obesity. Archives of Pediatrics and Adolescent Medicine. 2003;157(8):739–745. https://www.ncbi.nlm.nih.gov/pubmed/12912778[↩]
- Vieweg WV, Julius DA, Benesek J, et al. Posttraumatic stress disorder and body mass index in military veterans. Preliminary findings. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2006;30(6):1150–1154. https://www.ncbi.nlm.nih.gov/pubmed/16647795[↩]
- Takaya K, Ariyasu H, Kanamoto N, Iwakura H, Yoshimoto A, Harada M, Mori K, Komatsu Y, Usui T, Shimatsu A, Ogawa Y, Hosoda K, Akamizu T, Kojima M, Kangawa K, Nakao K. Ghrelin strongly stimulates growth hormone (GH) release in humans. J Clin Endocrinol Metab 2000; 85: 4908–4911. https://www.ncbi.nlm.nih.gov/pubmed/11134161[↩]
- Arvat E, Maccario M, Di Vito L, Broglio F, Benso A, Gottero C, Papotti M, Muccioli G, Dieguez C, Casanueva FF, Deghenghi R, Camanni F, Ghigo E. Endocrine activities of ghrelin, a natural growth hormone secretagogue (GHS), in humans: comparison and interactions with hexarelin, a nonnatural peptidyl GHS, and GH-releasing hormone. J Clin Endocrinol Metab 2001; 86: 1169–1174. https://www.ncbi.nlm.nih.gov/pubmed/11238504[↩]
- Obesity Reviews Volume 12, Issue 5, May 2011. Pages e211–e218. http://onlinelibrary.wiley.com/doi/10.1111/j.1467-789X.2010.00776.x/full[↩]
- Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body Fatness and Cancer–Viewpoint of the IARC Working Group. New England Journal of Medicine 2016; 375(8):794-798. doi: 10.1056/NEJMsr1606602[↩]
- Setiawan VW, Yang HP, Pike MC, et al. Type I and II endometrial cancers: have they different risk factors? Journal of Clinical Oncology 2013; 31(20):2607-2618. https://www.ncbi.nlm.nih.gov/pubmed/23733771[↩]
- Dougan MM, Hankinson SE, Vivo ID, et al. Prospective study of body size throughout the life-course and the incidence of endometrial cancer among premenopausal and postmenopausal women. International Journal of Cancer 2015; 137(3):625-37. https://www.ncbi.nlm.nih.gov/pubmed/25641700[↩]
- Hoyo C, Cook MB, Kamangar F, et al. Body mass index in relation to oesophageal and oesophagogastric junction adenocarcinomas: a pooled analysis from the International BEACON Consortium. International Journal of Epidemiology 2012; 41(6):1706-1718. https://www.ncbi.nlm.nih.gov/pubmed/23148106[↩]
- Chen Y, Liu L, Wang X, et al. Body mass index and risk of gastric cancer: a meta-analysis of a population with more than ten million from 24 prospective studies. Cancer Epidemiology, Biomarkers & Prevention 2013; 22(8):1395-1408. https://www.ncbi.nlm.nih.gov/pubmed/23697611[↩]
- Chen Y, Wang X, Wang J, Yan Z, Luo J. Excess body weight and the risk of primary liver cancer: an updated meta-analysis of prospective studies. European Journal of Cancer 2012; 48(14):2137-2145. https://www.ncbi.nlm.nih.gov/pubmed/22446023[↩]
- Campbell PT, Newton CC, Freedman ND, et al. Body mass index, waist circumference, diabetes, and risk of liver cancer for U.S. adults. Cancer Research 2016; 76(20):6076-6083. https://www.ncbi.nlm.nih.gov/pubmed/27742674[↩]
- Wang F, Xu Y. Body mass index and risk of renal cell cancer: a dose-response meta-analysis of published cohort studies. International Journal of Cancer 2014; 135(7):1673-86. https://www.ncbi.nlm.nih.gov/pubmed/24615287[↩]
- Sanfilippo KM, McTigue KM, Fidler CJ, et al. Hypertension and obesity and the risk of kidney cancer in 2 large cohorts of US men and women. Hypertension 2014; 63(5):934-41. https://www.ncbi.nlm.nih.gov/pubmed/24637660[↩]
- Wallin A, Larsson SC. Body mass index and risk of multiple myeloma: a meta-analysis of prospective studies. European Journal of Cancer 2011; 47(11):1606-1615. https://www.ncbi.nlm.nih.gov/pubmed/21354783[↩]
- Niedermaier T, Behrens G, Schmid D, et al. Body mass index, physical activity, and risk of adult meningioma and glioma: A meta-analysis. Neurology 2015; 85(15):1342-1350. https://www.ncbi.nlm.nih.gov/pubmed/26377253[↩]
- Genkinger JM, Spiegelman D, Anderson KE, et al. A pooled analysis of 14 cohort studies of anthropometric factors and pancreatic cancer risk. International Journal of Cancer 2011; 129(7):1708-1717. https://www.ncbi.nlm.nih.gov/pubmed/21105029[↩]
- Ma Y, Yang Y, Wang F, et al. Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS One 2013; 8(1):e53916. https://www.ncbi.nlm.nih.gov/pubmed/23349764[↩][↩]
- World Cancer Research Fund International/American Institute for Cancer Research. Continuous Update Project Report: Diet, Nutrition, Physical Activity and Gallbladder Cancer. 2015. http://www.wcrf.org/sites/default/files/Gallbladder-Cancer-2015-Report.pdf[↩]
- Li L, Gan Y, Li W, Wu C, Lu Z. Overweight, obesity and the risk of gallbladder and extrahepatic bile duct cancers: A meta-analysis of observational studies. Obesity (Silver Spring) 2016; 24(8):1786-1802. https://www.ncbi.nlm.nih.gov/pubmed/27392541[↩]
- Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008; 371(9612):569-578. https://www.ncbi.nlm.nih.gov/pubmed/18280327[↩]
- Munsell MF, Sprague BL, Berry DA, Chisholm G, Trentham-Dietz A. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiologic Reviews 2014; 36:114-136. www.ncbi.nlm.nih.gov/pubmed/24375928[↩][↩]
- Brinton LA, Cook MB, McCormack V, et al. Anthropometric and hormonal risk factors for male breast cancer: male breast cancer pooling project results. Journal of the National Cancer Institute 2014; 106(3):djt465. https://www.ncbi.nlm.nih.gov/pubmed/24552677[↩]
- Collaborative Group on Epidemiological Studies of Ovarian Cancer. Ovarian cancer and body size: individual participant meta-analysis including 25,157 women with ovarian cancer from 47 epidemiological studies. PLoS Medicine 2012; 9(4):e1001200. https://www.ncbi.nlm.nih.gov/pubmed/22606070[↩][↩]
- Kitahara CM, McCullough ML, Franceschi S, et al. Anthropometric factors and thyroid cancer risk by histological subtype: Pooled analysis of 22 prospective studies. Thyroid 2016; 26(2):306-318. https://www.ncbi.nlm.nih.gov/pubmed/26756356[↩]
- Keum N, Greenwood DC, Lee DH, et al. Adult weight gain and adiposity-related cancers: a dose-response meta-analysis of prospective observational studies. Journal of the National Cancer Institute 2015; 107(2). pii: djv088. https://www.ncbi.nlm.nih.gov/pubmed/25757865[↩]
- Tee MC, Cao Y, Warnock GL, Hu FB, Chavarro JE. Effect of bariatric surgery on oncologic outcomes: a systematic review and meta-analysis. Surgical Endoscopy 2013; 27(12):4449-4456. https://www.ncbi.nlm.nih.gov/pubmed/23949484[↩]
- Neuhouser ML, Aragaki AK, Prentice RL, et al. Overweight, obesity, and postmenopausal invasive breast cancer risk: A secondary analysis of the Women’s Health Initiative randomized clinical trials. JAMA Oncology 2015; 1(5):611-621. www.ncbi.nlm.nih.gov/pubmed/26182172[↩]
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. New England Journal of Medicine 2003; 348(17):1625-1638. http://www.nejm.org/doi/full/10.1056/NEJMoa021423[↩]
- Schmitz KH, Neuhouser ML, Agurs-Collins T, et al. Impact of obesity on cancer survivorship and the potential relevance of race and ethnicity. Journal of the National Cancer Institute 2013; 105(18):1344-1354. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3776266/[↩]
- Paskett ED, Dean JA, Oliveri JM, Harrop JP. Cancer-related lymphedema risk factors, diagnosis, treatment, and impact: a review. Journal of Clinical Oncology 2012; 30(30):3726-3733. https://www.ncbi.nlm.nih.gov/pubmed/23008299[↩]
- Gacci M, Sebastianelli A, Salvi M, et al. Role of abdominal obesity for functional outcomes and complications in men treated with radical prostatectomy for prostate cancer: results of the Multicenter Italian Report on Radical Prostatectomy (MIRROR) study. Scandinavian Journal of Urology 2014; 48(2):138-145. https://www.ncbi.nlm.nih.gov/pubmed/23781856[↩]
- Meyerhardt JA, Tepper JE, Niedzwiecki D, et al. Impact of body mass index on outcomes and treatment-related toxicity in patients with stage II and III rectal cancer: findings from Intergroup Trial 0114. Journal of Clinical Oncology 2004; 22(4):648-657. https://www.ncbi.nlm.nih.gov/pubmed/14966087[↩]
- Teras LR, Kitahara CM, Birmann BM, et al. Body size and multiple myeloma mortality: a pooled analysis of 20 prospective studies. British Journal of Haematology 2014; 166(5):667-676. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4134758/[↩]
- Goodwin PJ, Segal RJ, Vallis M, et al. Randomized trial of a telephone-based weight loss intervention in postmenopausal women with breast cancer receiving letrozole: the LISA trial. Journal of Clinical Oncology 2014; 32(21):2231-2239. https://www.ncbi.nlm.nih.gov/pubmed/24934783[↩]
- Harrigan M, Cartmel B, Loftfield E, et al. Randomized trial comparing telephone versus in-person weight loss counseling on body composition and circulating biomarkers in women treated for breast cancer: The Lifestyle, Exercise, and Nutrition (LEAN) Study. Journal of Clinical Oncology 2016; 34(7):669-676. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4872022/[↩]
- Goodwin PJ. Obesity and breast cancer outcomes: How much evidence is needed to change practice? Journal of Clinical Oncology 2016; 34(7):646-648. Epub 2015 Dec 28. http://ascopubs.org/doi/full/10.1200/JCO.2015.64.7503[↩]
- Gallagher EJ, LeRoith D. Obesity and diabetes: The increased risk of cancer and cancer-related mortality. Physiological Reviews 2015; 95(3):727-748. www.ncbi.nlm.nih.gov/pubmed/26084689[↩]
- Sheflin AM, Whitney AK, Weir TL. Cancer-promoting effects of microbial dysbiosis. Current Oncology Reports 2014; 16(10):406. https://www.ncbi.nlm.nih.gov/pubmed/25123079[↩]
- Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015; 350(6264):1084-1089. www.ncbi.nlm.nih.gov/pubmed/26541606[↩]
- Vétizou M, Pitt JM, Daillère R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015; 350(6264):1079-1084. https://www.ncbi.nlm.nih.gov/pubmed/26541610[↩]
- Barrington WE, Schenk JM, Etzioni R, et al. Difference in association of obesity with prostate cancer risk between US African American and non-Hispanic white men in the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA Oncology 2015; 1(3):342-349. https://www.ncbi.nlm.nih.gov/pubmed/26181184[↩]
- Obes Rev. 2008 Sep;9(5):474-88. doi: 10.1111/j.1467-789X.2008.00475.x. Epub 2008 Mar 5. Tracking of childhood overweight into adulthood: a systematic review of the literature. https://www.ncbi.nlm.nih.gov/pubmed/18331423[↩]
- BMJ 2012;345:e4759. https://doi.org/10.1136/bmj.e4759. Cardiovascular disease risk in healthy children and its association with body mass index: systematic review and meta-analysis. http://www.bmj.com/content/345/bmj.e4759[↩][↩][↩][↩]
- S. S. Sun, G. D. Grave, R. M. Siervogel, A. A. Pickoff, S. S. Arslanian, and S. R. Daniels, “Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life,” Pediatrics, vol. 119, no. 2, pp. 237–246, 2007.[↩]
- R. H. Eckel, S. M. Grundy, and P. Z. Zimmet, “The metabolic syndrome,” The Lancet, vol. 365, no. 9468, pp. 1415–1428, 2005.[↩]
- S. Redline, A. Storfer-Isser, C. L. Rosen et al., “Association between metabolic syndrome and sleep-disordered breathing in adolescents,” American Journal of Respiratory and Critical Care Medicine, vol. 176, no. 4, pp. 401–408, 2007.[↩]
- BMC Pediatrics 2013, 13:121. DOI: 10.1186/1471-2431-13-121. Childhood body mass index and subsequent physician-diagnosed asthma: a systematic review and meta-analysis of prospective cohort studies. https://bmcpediatr.biomedcentral.com/articles/10.1186/1471-2431-13-121[↩]
- Journal of Nutrition and Metabolism Volume 2012 (2012), Article ID 134202, 8 pages. Childhood Obesity and Obstructive Sleep Apnea. https://www.hindawi.com/journals/jnme/2012/134202/[↩]
- M. Tsaoussoglou, E. O. Bixler, S. Calhoun, G. P. Chrousos, K. Sauder, and A. N. Vgontzas, “Sleep-disordered breathing in obese children is associated with prevalent excessive daytime sleepiness, inflammation, and metabolic abnormalities,” The Journal of Clinical Endocrinology and Metabolism, vol. 95, no. 1, pp. 143–150, 2010.[↩]
- K. A. Waters, S. Sitha, L. M. O’Brien et al., “Follow-up on metabolic markers in children treated for obstructive sleep apnea,” American Journal of Respiratory and Critical Care Medicine, vol. 174, no. 4, pp. 455–460, 2006.[↩]
- Int J Pediatr Obes. 2010 Aug;5(4):282-304. doi: 10.3109/17477160903473697. Self-esteem and quality of life in obese children and adolescents: a systematic review. https://www.ncbi.nlm.nih.gov/pubmed/20210677[↩]
- The Conversation June 22, 2017. What’s the best way for children to lose weight? Here’s what the research says. https://theconversation.com/whats-the-best-way-for-children-to-lose-weight-heres-what-the-research-says-79714[↩]
- Cochrane Review 22 June 2017. Diet, physical activity and behavioural interventions for the treatment of overweight or obese children from the age of 6 to 11 years. http://www.cochrane.org/CD012651/ENDOC_diet-physical-activity-and-behavioural-interventions-treatment-overweight-or-obese-children-age-6-11[↩][↩][↩][↩][↩][↩]
- Cochrane Review 22 June 2017. Diet, physical activity and behavioural interventions for the treatment of overweight or obese adolescents aged 12 to 17 years. http://www.cochrane.org/CD012691/ENDOC_diet-physical-activity-and-behavioural-interventions-treatment-overweight-or-obese-adolescents-aged[↩][↩][↩][↩][↩][↩]
- Wareham NJ, van Sluijs EM, Ekelund U. Physical activity and obesity prevention: a review of the current evidence. Proc Nutr Soc. 2005; 64:229-47. https://www.ncbi.nlm.nih.gov/pubmed/15960868[↩]
- Seo DC, Li K. Leisure-time physical activity dose-response effects on obesity among US adults: results from the 1999-2006 National Health and Nutrition Examination Survey. J Epidemiol Community Health. 2010; 64:426-31. http://jech.bmj.com/content/64/5/426.long[↩]
- Lewis CE, Smith DE, Wallace DD, Williams OD, Bild DE, Jacobs DR, Jr. Seven-year trends in body weight and associations with lifestyle and behavioral characteristics in black and white young adults: the CARDIA study. Am J Public Health. 1997; 87:635-42. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1380845/pdf/amjph00503-0109.pdf[↩]
- Lee IM, Djousse L, Sesso HD, Wang L, Buring JE. Physical activity and weight gain prevention. JAMA. 2010; 303:1173-9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2846540/[↩]
- Lusk AC, Mekary RA, Feskanich D, Willett WC. Bicycle riding, walking, and weight gain in premenopausal women. Arch Intern Med. 2010; 170:1050-6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3119355/[↩]
- Mekary RA, Feskanich D, Malspeis S, Hu FB, Willett WC, Field AE. Physical activity patterns and prevention of weight gain in premenopausal women. Int J Obes (Lond). 2009; 33:1039-47. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2746452/[↩]
- Mekary RA, Feskanich D, Hu FB, Willett WC, Field AE. Physical activity in relation to long-term maintenance after intentional weight loss in premenopausal women. Obesity (Silver Spring, Md). 2010;18(1):167-174. doi:10.1038/oby.2009.170. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2798010/[↩]
- U.S. Dept. of Health and Human Services. 2008 Physical Activity Guidelines for Americans; 2008. https://health.gov/paguidelines/[↩]
- Slentz CA, Aiken LB, Houmard JA, et al. Inactivity, exercise, and visceral fat. STRRIDE: a randomized, controlled study of exercise intensity and amount. J Appl Physiol. 2005; 99:1613-8. https://www.physiology.org/doi/pdf/10.1152/japplphysiol.00124.2005[↩][↩][↩]
- McTiernan A, Sorensen B, Irwin ML, et al. Exercise effect on weight and body fat in men and women. Obesity (Silver Spring). 2007; 15:1496-512. https://onlinelibrary.wiley.com/doi/pdf/10.1038/oby.2007.178[↩]
- Friedenreich CM, Woolcott CG, McTiernan A, et al. Adiposity changes after a 1-year aerobic exercise intervention among postmenopausal women: a randomized controlled trial. Int J Obes (Lond). 2010. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3061001/[↩][↩]
- Sallis JF, Glanz K. Physical activity and food environments: solutions to the obesity epidemic. Milbank Q. 2009; 87:123-54. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2879180/[↩]
- Physical Activity Guidelines for Americans. https://health.gov/paguidelines/[↩][↩][↩][↩][↩][↩][↩][↩]
- Lee, I.M., et al., Physical activity and weight gain prevention. JAMA, 2010. 303(12): p. 1173-9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2846540/[↩][↩]
- Jakicic, J.M., et al., Effect of exercise duration and intensity on weight loss in overweight, sedentary women: a randomized trial. JAMA, 2003. 290(10): p. 1323-30. https://www.ncbi.nlm.nih.gov/pubmed/12966123[↩]
- Williams, M.A., et al., Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation, 2007. 116(5): p. 572-84. http://circ.ahajournals.org/content/116/5/572.long[↩][↩]
- Schmitz, K.H., et al., Strength training and adiposity in premenopausal women: strong, healthy, and empowered study. Am J Clin Nutr, 2007. 86(3): p. 566-72. https://www.ncbi.nlm.nih.gov/pubmed/17823418[↩]
- Engelke, K., et al., Exercise maintains bone density at spine and hip EFOPS: a 3-year longitudinal study in early postmenopausal women. Osteoporos Int, 2006. 17(1): p. 133-42.[↩]
- Ling, C.H., et al., Handgrip strength and mortality in the oldest old population: the Leiden 85-plus study. CMAJ, 2010. 182(5): p. 429-35. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2842834[↩]
- Ruiz, J.R., et al., Association between muscular strength and mortality in men: prospective cohort study. BMJ, 2008. 337: p. a439. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2453303/[↩]
- Bolam, K.A., J.G. van Uffelen, and D.R. Taaffe, The effect of physical exercise on bone density in middle-aged and older men: A systematic review. Osteoporos Int, 2013. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0055053/[↩][↩]
- Nelson, M.E., et al., Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation, 2007. 116(9): p. 1094-105. http://circ.ahajournals.org/content/116/9/1094.long[↩][↩]
- N Engl J Med. 2009 Feb 26;360(9):859-73. doi: 10.1056/NEJMoa0804748. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. https://www.ncbi.nlm.nih.gov/pubmed/19246357[↩][↩][↩]
- Manheimer EW, van Zuuren EJ, Fedorowicz Z, Pijl H. Paleolithic nutrition for metabolic syndrome: systematic review and meta-analysis. The American Journal of Clinical Nutrition. 2015;102(4):922-932. doi:10.3945/ajcn.115.113613. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4588744[↩]
- https://www.niddk.nih.gov/[↩]
- Fothergill E, Guo J, Howard L, Hall KD. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity 24: 1612-1619, 2016.[↩]
- N Engl J Med. 2010 Nov 25;363(22):2102-13. doi: 10.1056/NEJMoa1007137. Diets with high or low protein content and glycemic index for weight-loss maintenance. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3359496/[↩][↩]
- Gluck ME, Alonso-Alonso M, Piaggi P, Krakoff J. Neuromodulation targeted to the prefrontal cortex induces changes in energy intake and weight loss in obesity. Obesity 23: 2149-2156, 2015.[↩]
- Lee J, Liu J, Feng X, Ozcan U. Withaferin A is a leptin sensitizer with strong antidiabetic properties in mice. Nat Med 22: 1023-1032, 2016.[↩]
- Tharp KM, Jha AK, Kraiczy J, Stahl A. Matrix-assisted transplantation of functional beige adipose tissue. Diabetes 64: 3713-3724, 2015. [↩]








