The universe is awash in extremes. The fastest, the oldest, the furthest, there are all kinds of things you can look into and be amazed by what nature has created. But what about the coldest? You’ve probably heard of absolute zero before, but do you know what happens when you reach it? Or why nothing can get colder?
The basic concept of absolute zero was discovered all the way back in 1702. French inventor Guillaume Amontons theorized that there had to be the lowest temperature that could be achieved when he realized air pressure and temperature were related. His experiments led him to believe there had to be a point when pressure could not get any lower and, therefore, temperature could not get any lower.
Amontons came up with a guess of -240 C for his lowest possible temperature. Given that he didn’t have a concept of the movement of atoms and his guess came in the early 1700s, it’s pretty impressive that he got so close to the mark.
It wasn’t until 1848 when physicist William Thomson, better known as Lord Kelvin, narrowed it down with some precision and gave us his eponymous Kelvin scale. Part of his motivation was to make an absolute temperature scale that made sense and ensured we didn’t need to use negative numbers. When you start at zero, you have nowhere to go but up.
Of course, for most of us in everyday life, the Kelvin scale just seems impractical and unwieldy. The Celsius scale does make more sense, after all, since it’s based on when water boils and freezes, which are touchstones that make sense to average folks.
Lucky for us, scientists play with extreme temperatures all the time just for kicks, so there’s a good deal of research into this topic. Grab a warm drink and let’s check it out.
Absolute Zero Physics

Because humans have a weird history of measuring things like feet and cords and acres and such, it took some time before we developed anything that was consistent or logical. Celsius and Fahrenheit had their day in the sun but the Kelvin scale is the one that takes things seriously. Absolute Zero means 0 Kelvin, the lowest possible temperature you can get to. That works out to -273 C or -459.67 F.
Cold is the relative property of any matter related to the movement of its atoms. This is why it has a limit, why you can achieve the lowest possible temperature of absolute zero. That’s the point at which the atoms in matter stop moving altogether, or as close to it as they’ll ever get, and its thermodynamic system has the lowest possible energy it can ever have.
This doesn’t necessarily mean that the atoms have stopped moving entirely at absolute zero, just that the energy of the atoms cannot be transferred at all. No heat, even the tiniest, barely measurable amount is produced. Because heat comes from the movement of atoms, and those atoms transfer that energy into other atoms they contact, there is no heat transfer at absolute zero.
For most of us, absolute zero is essentially trivia and nothing else. We’re never going to discover it in real life and we certainly wouldn’t have much time to appreciate it if we did since it would kill us awfully quickly.
In lab conditions where absolute zero has been nearly achieved, scientists have observed remarkable changes in physics. For instance, at super low temperatures some substances will flow uphill instead of downhill in defiance of gravity. Chemical reactions also have to bend to the will of cold.
At absolute zero any chemical reaction would theoretically have to stop dead in its tracks by definition. Nearing absolute zero, scientists can dramatically slow reactions that would normally take place in fractions of a second, allowing them to observe and even manipulate how those reactions occur.
Science at super-low temperatures offers a glimpse into things like quantum mechanics that we otherwise wouldn’t have operating at normal temperatures. That’s why this field is so interesting to scientists, because it’s allowing them to learn and observe some of the fundamental ways particles and reactions occur in the universe but in a way that we can actually see.
The Coldest Place in the Universe
We all know that the depths of space are extremely cold but when we’re talking absolute zero, it’s positively balmy out there. The deepest, darkest reaches of space are 2.7 Kelvin. Admittedly, that will kill any living thing super dead since it’s -270.45 C or -453.8F, but it’s still a ways off from absolute zero.
Space is not uniform in temperature, of course. Stars like our sun do warm things up when you get close, so you need to find a real middle-of-nowhere spot to get those low lows.
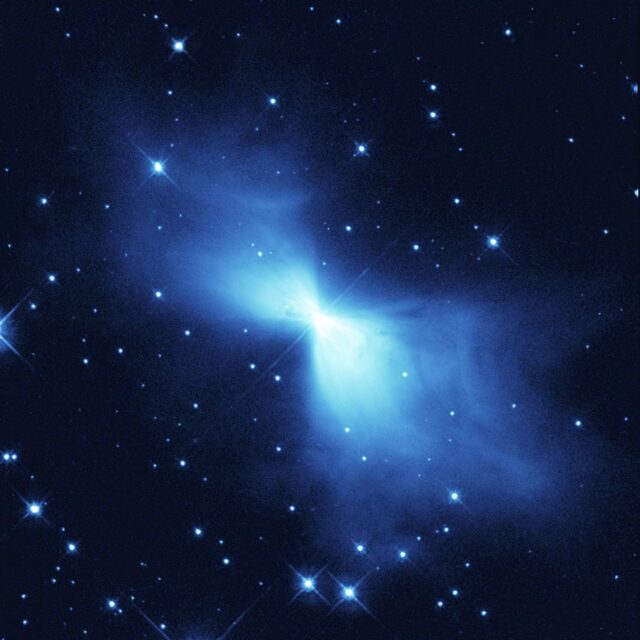
5,000 light years from Earth you’ll find something called the Boomerang Nebula. It’s made of dust and ionizing gasses and it’s about the coldest natural place you’re ever likely to find. A red giant is dying in the middle of the nebula and because it’s shedding all of its stellar material at an astronomically fast rate, about 250 times faster than an F-16 fighter jet, it’s losing heat in a dramatic way as well.
The Nebula’s temperature has been measured at about a degree Celsius above absolute zero. You can contrast that with the lowest recorded temperature recorded on Earth which dates back to 1983 and clocked in at -89.2C or -128.6F. That’s a scorching 183.95 Kelvin. That temperature was recorded at the Vostok station in Antarctica. That means the Nebula is about three times colder than Earth has ever been, which sounds a lot less terrifying than it really is.
Can We Make Absolute Zero in a Lab?
One fun thing about absolute zero is that scientists have managed to get pretty close to it in lab conditions. Some scientific concepts have to remain extremely theoretical. No one has managed to produce a black hole yet or develop time travel. But if you want absolute zero, the best we’ve managed to get so far is 38 picokelvins, which is 38 trillionths of a degree above absolute zero. It was achieved by dropping magnetized rubidium gas down a tower in a German lab which is how you make really cold things these days.
While 38 trillionths sounds incredibly close, odds are we’re not going to ever get much closer. It is generally believed that absolute zero can never truly be achieved thanks to quantum fluctuations. Thermodynamics also doesn’t allow for one to reach the absolute lowest temperature, either, and we’ll touch on that a little bit later on.
As far as humans are concerned, another roadblock is flawed technology. At some point, obviously beyond 38 trillionths of a degree, we simply can’t measure with enough accuracy to say for certain we have achieved perfect, absolute zero. Absolute zero and the barest fraction above it would be indistinguishable from our instruments.
The problem with instruments is so significant that it’s also been suggested, even if we could reach absolute zero, we might not even realize we did it because of the potential inaccuracy. You would need a thermometer that could measure temperature with infinite accuracy and that is quite obviously something that can’t exist, so we could never precisely get the measurements we need.
Fortunately, many scientists who deal in this realm of research don’t actually want to achieve absolute zero. It’s those near absolute zero temperatures that are the most interesting because they still allow for some very minor atomic movement. Reactions can still be observed whereas, if absolute zero was achieved, there would be essentially nothing to look at. It’s the physics version of a lot of buildup with no payoff.
While absolute zero works as a great concept it’s stuck in the realm of the theoretical, though some would still debate it could technically be reached. Science doesn’t always agree on that point, however.
Why It’s Impossible
Neil DeGrasse Tyson offered some explanations for why reaching absolute zero is actually impossible. His first argument is one of heat exchange and he used the example of a refrigerator. You put room-temperature food in the fridge and the cold air in the fridge draws the heat from the food. Heat is a thing, after all, but cold is just the absence of it. You take that heat energy away from something and it becomes cold; you don’t have to add anything to it.
So the colder air in a fridge takes heat from warmer food until you reach the overall temperature of your fridge, freezer, or wherever the thing losing heat is located. But on a Kelvin scale, you hit a problem with absolute zero. To take that last bit of heat from something to reach absolute zero, you need something colder than absolute zero (this all starts around the 8:20 mark in the video) to do it. You need something colder than can draw that last nearly unmeasurable amount of heat away. But how could that ever exist?
Tyson goes on to explain a second problem. According to Quantum Physics, you can never know exactly where the atoms in a particle are. Quantum vibration has to exist all the time allowing those atoms to vibrate, even a tiny bit. There would have to be a Quantum Limit to absolute zero that prevents the atoms from stopping entirely because, if they did, you could pinpoint any given atom and that just doesn’t work with physics.
In 1912, it was theorized that absolute zero would be impossible to reach on a finite timeline. This is known as the Unattainability Principle. This relates to Tyson’s first point about finding a way to take away that final fraction of a degree of heat from something to achieve absolute zero.
It’s been shown in experiments that, to cool something to absolute zero, one requires either infinite work or an infinitely big cooling reservoir. As something approaches absolute zero, so too does its entropy approach zero and, according to physics, it’s impossible to prepare a system in a state of zero entropy in a finite number of steps.
One experiment has determined that absolute zero could be theoretically attainable but there are three ingredients required for this to happen. One is time, another is complexity, and the third one is energy. The problem that this experiment presented is that, in order to obtain absolute zero, you need to have one of those ingredients in infinite quantities. As long as you can do that, you could achieve your goal. You may recognize this as another way of saying that it is impossible to achieve.
What Would Theoretically Happen?
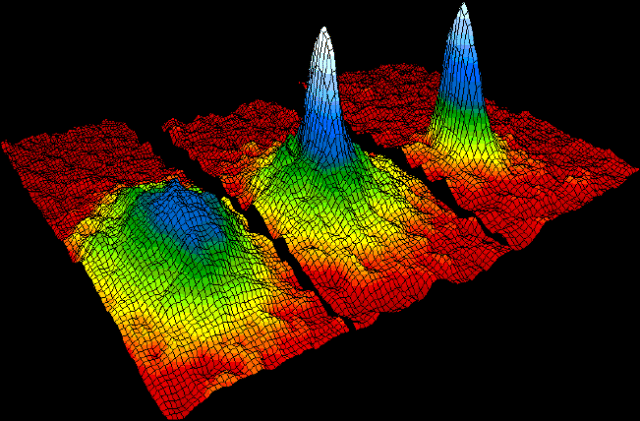
One thing we know that happens at ultra-low temperatures, bordering absolute zero, is the formation of a Bose-Einstein Condensate or BEC. A BEC is considered another state of matter but it only exists at these ultra-low temperatures. It occurs when a gas is super-cooled and the atoms or subatomic particles merge into a quantum mechanical entity. That sounds very sci-fi, but it essentially means that the atoms operate as a wave function all together, like they have just become one super-atom.
Einstein, using formulas from Bose, theorized this would happen many decades before it was actually proven, but it follows the rules of quantum mechanics. It can only happen at low temperatures, however, because the individual atoms need to be operating at such low energy that they begin to move together.
If we were able to push past this Bose-Einstein Condensate state and hit true, absolute zero, then matter would lose all of its kinetic energy. If there was no kinetic energy and the atoms were frozen in place, the thing you were observing could no longer be considered matter. As of right now, physics as we understand it does not allow for matter to simply no longer be matter.