Preprint
Review
A Comprehensive Review of Natural Product as Therapeutic Agent for Head and Neck Squamous Cell Carcinoma Using Preclinical Model
Altmetrics
Downloads
134
Views
42
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Abstract
Head and neck squamous cell carcinoma (HNSCC) is a type of cancer that arises from the epithelium lining of the oral cavity, hypopharynx, oropharynx, and larynx. Despite advancement of the current treatment to HNSCC, such as surgery, chemotherapy, and radiotherapy, the overall survival rate of HNSCC remains poor due to late diagnosis and acquired resistance to treatment. Natural products have been extensively explored as a safer and more acceptable alternative to current treatments, with numerous studies displaying their potential against HNSCC. This review highlights preclinical studies in the past 5 years involving natural products against HNSCC and explores the signalling pathways altered by these products. This review also addresses challenges and future directions of the use of natural products as chemotherapeutic and chemoprevention agents against HNSCC.
Keywords:
Submitted:
16 June 2023
Posted:
19 June 2023
You are already at the latest version
Alerts
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
16 June 2023
Posted:
19 June 2023
You are already at the latest version
Alerts
Abstract
Head and neck squamous cell carcinoma (HNSCC) is a type of cancer that arises from the epithelium lining of the oral cavity, hypopharynx, oropharynx, and larynx. Despite advancement of the current treatment to HNSCC, such as surgery, chemotherapy, and radiotherapy, the overall survival rate of HNSCC remains poor due to late diagnosis and acquired resistance to treatment. Natural products have been extensively explored as a safer and more acceptable alternative to current treatments, with numerous studies displaying their potential against HNSCC. This review highlights preclinical studies in the past 5 years involving natural products against HNSCC and explores the signalling pathways altered by these products. This review also addresses challenges and future directions of the use of natural products as chemotherapeutic and chemoprevention agents against HNSCC.
Keywords:
Subject: Biology and Life Sciences - Other
1. Introduction
Head and neck cancer (HNC) represents cancers occurred in the head and neck region, which includes the lip and oral cavity, nasal cavity, larynx and pharynx [1]. Among more than 90% of HNCs are head and neck squamous cell carcinomas (HNSCCs), which accounts for more than 300,000 deaths and 500,000 new cases worldwide annually [2]. In the United States, HNSCC has been diagnosed as one of the top 10 leading cancers in men in 2022 [2,3,4]. The low survival rate of HNSCC had been proposed to be associated with cancer recurrence, distant metastases, progression of second primary cancers and resistance to chemo/radiotherapy [5,6]. Making it as a public health issue that compromise patients’ quality of life.
Tobacco smoking, excessive alcohol, betel quid chewing and high-risk human papillomavirus (HPV) had been documented as risk factors for HNSCC [7,8,9,10]. Clinical intervention of HNSCC often took place at an advanced stages of the disease due to late diagnosis and poor prognosis, especially among individuals with a lower socioeconomical background [11,12]. The most common clinical intervention for HNSCC are surgery, radiotherapy, chemotherapy, or combined therapy which causes numerous side effects while providing some therapeutic effects during the treatment of this disease [11]. However, even with a successful clinical intervention, approximately 30% of patients treated at an advanced stages of the disease would develop recurrent locoregional or second primary cancers, with the onset of chemo-resistance or radio-resistance and treatment failure as the most prominent underlying factor [13,14,15].
Cetuximab is an epidermal growth factor receptor (EGFR) targeting monoclonal antibody, which is also the first molecular-targeted drug and received approval by U.S. Food and Drug Administration (FDA) as chemotherapeutic agent for HNSCC in 2006 [5,16]. A retrospective study in Japan had reported a total effective rate of 57.1%, median progression-free survival (PFS) of 5.5 months and overall survival (OS) of 8.0 months with cetuximab in locally advanced HNSCC, while a total effective rate of 60.0%, PFS of 3.8 months and OS of 5.8 months in distantly metastatic HNSCC [17]. However, chemoresistance ability of certain mutated cancer cell type such as EGFRvIII showed resistance towards cetuximab, making the drug non-effective as a therapeutic agent towards HNSCC [18]. In the recent years, pembrolizumab and nivolumab, which both act as anti-programmed cell death receptor 1 (PD-1) immunotherapy drugs had been approved by FDA for recurrent or metastatic HNSCC treatment [19,20]. However, most patient previously exposed to the anti-PD-1 monoclonal antibody would develop acquired resistance to immunotherapeutic drug, making it difficult to treat recurrent or metastatic cancers [21]. Therefore, there is an urgent need for alternative therapeutic agents to overcome the acquired resistance of HNSCC to standard of care. Meanwhile, as therapeutic approaches such as surgical intervention, radiotherapy and chemotherapy are only effective against a limited subgroup of HNSCC patients, and often resulted in additional morbidities, there is a great need for prevention for high-risk potentially malignant lesions and agents that can work effectively as chemoprevention agents or to enhance the effectiveness of chemotherapeutic agents when combined to kill cancer cell.
Natural products are compounds naturally found in natural resources such as plants which possesses biological activities [22]. In the recent years, natural products had been widely reported for their chemotherapeutic and chemoprevention properties against HNSCC, due to their low cytotoxicity, efficacy against cancers, availability and low cost [12,22]. According to National Cancer Institute, U.S., chemotherapy is defined as the treatment that uses drugs to stop the growth of cancer cells, either by killing the cells or by stopping them from dividing, while chemoprevention is defined as the use of certain drugs or other substances to help lower a person’s risk of developing cancer or keep it from coming back [23]. The beneficial properties of natural products are consistent and could overcome the challenges with current treatment such as acquired chemoresistance, cytotoxicity against normal cells and expensive therapy. Numerous studies had been carried out with various natural products on preclinical models of HNSCC including various HNSCC cell lines and xenograft or carcinogen induced-tumor animal model (Figure 1). For example, psorachromene, a flavonoid found in Psoralea corylifolia, which had been used in traditional Chinese medicine (TCM) and Ayurvedic had shown therapeutic affects against HNSCC via regulation of EGFR signaling pathways, and other carcinogenesis-related signaling pathway, making it a strong candidate to act as a chemotherapeutic agent [24]. Other natural products such as calcitrol, had been reported to possess chemoprevention properties against carcinogen induced HNSCC in animal models [25]. Therefore, natural products could potentially act as adjuvant or neoadjuvant chemotherapy, and chemoprevention of HNSCC.
Activation of complex signaling pathways such as phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/Akt/mTOR) and mitogen-activated protein kinase/extracellular signal-regulated protein kinase (MAPK/ERK) are important for tumor development, cell survival and angiogenesis, while epithelial to mesenchymal transition (EMT) signaling leads to tumor invasion and migration [26,27]. Thus, inhibition of the signaling pathway could lead to cell death, inhibiting the tumor development, and inhibiting metastasis [27]. Notwithstanding the extensive knowledge at the molecular level of tumor-associated signaling pathways, the chemotherapeutic agents targeting the oncogenic pathways are limited [28]. To date, Crooker et al. and Rahman et al. have described the use of natural products as chemoprevention agents against HNSCC [29,30]. Nevertheless, several natural products such as vitamin A, green tea extract and curcumin which had shown promising result in preclinical studies were reported to show toxicity and limited bioavailability in clinical studies [31,32,33,34]. Vitamin A has been shown by Shin et al. [31] and Papadimitrakopoulou et al. [33] with clinical trials to induce toxicity, majority in terms of oral mucosa and lip inflammation, conjunctivitis, skin reactions, fatigue, joint pain and muscle pain. On the other hand, poor oral absorption of green tea extract and curcumin were observed which limits the bioavailability of both natural products [33,34]. Other emerging chemotherapeutic phytochemicals or herbal derivatives against HNC has recently been described by Aggarwal et al. [12]. However, several newly emerged phytochemicals against or preventing HNSCC, such as actein, calcitriol and psorachromene were not fully addressed. Therefore, in this review, the comprehensive mechanisms of various natural products showing significant preclinical results in HNSCC models in the past 5 years will be discussed.
2. Chemotherapeutic Properties of Natural Products Against Essential Pathway for HNSCC
2.1. PI3K/Akt/mTOR Pathway
It was previously reported that the activation of PI3K/Akt/mTOR pathway has been observed in approximately 90% of HNSCC, making it a prominent target for treatments of HNSCC [35,36]. The activation of PI3K/Akt/mTOR signaling also played an important role in HNSCC chemotherapy and radiotherapy resistances, which inhibition of the signaling pathway had shown positive effects on tumour proliferation and radiotherapy sensitization in preclinical studies [37,38]. PI3K/Akt/mTOR pathway are activated when ligand-like growth factor binds with the receptor tyrosine kinase (RTK), leading to activation of PI3K which in turn partially activating Akt [39]. mTORC2 is then required to completely activate Akt via phosphorylation, leading to activation of multiple proteins involved in cell proliferation and motility [40]. Inhibition of Akt formation will therefore limit the expression of oncoprotein. Various natural products, namely actein, salicylate, tanshinone IIA, xanthohumol, fucoidan, honokiol, ilimaquinone, nimbolide, and cinnamaldehyde had led to the downregulation of Akt expression and phosphorylation of Akt (p-Akt) using HNSCC preclinical models [41,42,43,44,45,46,47,48,49]. The downregulation of mTOR expression in HNSCC preclinical models had been shown by salicylate, honokiol, cinnamaldehyde, and Seco-A-ring oleanane, and PI3K would also limit the phosphorylation of Akt [42,45,48,50]. The expression of glycogen synthase kinase-3β (GSK-3β) plays an important role in regulating growth, cell cycle progression, apoptosis and cancer cell invasion in HNSCC [51,52]. The inactivation of GSK-3β via phosphorylation has previously shown significant inhibition in cancer cell growth and migration in HNSCC [51]. Preclinical studies with nimbolide had also shown upregulation of phosphorylated GSK-3β, inactivating GSK-3β, thus inhibiting cell proliferation [47]. The expression of c-Myc has been associated with upregulation of Akt-related pathways leading to cancer cell proliferation and tumourigenesis [53]. The downregulation of c-Myc using HNSCC preclinical model had been observed with tanshinone IIA, leading to inhibition of tumorigenesis [43].
2.2. MAPK/ERK Pathway
Activation of MAPK/ERK signaling pathway which includes signaling molecules such as interleukin-8 (IL-8), vascular endothelial growth factor (VEGF), mitogen-activated extracellular protein kinase (MEK) and extracellular signal-regulated protein kinase (ERK), is strongly associated with the expression of oncoproteins leading to cell proliferation and angiogenesis [54]. Honokiol, sodium Danshensu, cinnamaldehyde and protocatechuic acid had been reported to inhibit MAPK/ERK signaling in HNSCC preclinical model, thus inhibiting cell proliferation [45,48,55,56]. Among which protocatechuic acid inhibits MAPK/ERK signaling via activating c-Jun N-terminal kinase/p38 (JNK/p38) signaling pathway [56]. However, the expression of JNK plays a dual role, tumour suppressive and tumour progressive, in HNSCC due to the complex crosstalk between multiple signaling molecules and pathways [57]. Therefore, further understanding of the role of JNK and p38 in HNSCC is required to ensure the relevance of future clinical research. The overexpression of matrix metalloproteinases (MMPs), such as MMP-2 and MMP-9 has been associated to the cause progression and metastasis of HNSCC [58]. Fucoidan, sodium Danshensu, [(3β)-3-hydroxy-lup-20(29)-en-28-oic acid] and Seco-A-ring oleanane had been reported to downregulate MMP-2 [49,50,55]. While fucoidan and trichodermin downregulate MMP-9 in HNSCC preclinical models [49,56], inhibiting cancer cell metastasis. Expression of VEGF plays an important role in blood vessels formation, known as angiogenesis, which in turn promote cancer cell growth due to supply of nutrients via newly formed blood vessels [59,60]. It was also noticed that VEGF could be upregulated by human growth factor (HGF) through PI3K/Akt/mTOR and MAPK signaling pathway [61]. Preclinical studies of HNSCC had indicated that cinnamaldehyde, [(3β)-3-hydroxy-lup-20(29)-en-28-oic acid] and Seco-A-ring oleanane could downregulate VEGF, inhibiting angiogenesis [48,50].
2.3. NF- κB and STAT3 Transcription Factors
Nuclear factor-κB (NF-κB) is a protein complex consisting of five transcription factors, namely, RelA, RelB, c-Rel, NF-κB1 and NF-κB2, which had been reported to play an important role in cell proliferation and survival in HNSCC [62,63]. Preclinical studies with neferine, trichodermin, cinnamaldehyde and Seco-A¬-ring oleanane had demonstrated inhibition of NF-κB with HNSCC preclinical models, leading to apoptosis [48,50,64,65].
Expression of signal transducer and activator of transcription-3 (STAT3), a transcription factor from the STAT family, plays an important role in cell proliferation, survival and metastasis in HNSCC [66,67,68]. Phosphorylation of STAT3 (p-STAT3) has been proposed as a crucial reaction for complete activation of STAT3 which is regulated by p38, EGFR and janus kinases (JAKs) [69,70]. The activation of STAT3 would also led to various STAT3-dependent pathways such as interleukin-8/STAT3 (IL-8/STAT3) and EGFR/STAT3/SRY-box transcription factor 2 (EGFR/STAT3/SOX2) pathways which had been shown to play an important role in cancer stemness [71,72]. Preclinical studies in HNSCC with trichodermin, and Seco-A-ring oleanane had indicated downregulation of p-STAT3 [50,65], while psorachromene showed downregulation of EGFR, leading to apoptosis and inhibiting metastasis [24].
Actein, a biologically active compound found in rhizome of Cinicifuga foetida had been discovered to inhibit forkhead box O1 (FoxO1) by upregulate phosphorylation of FoxO1 (p-FoxO1), leading to inhibition of cell proliferation via Akt/FoxO1 signaling pathway [41]. It was also noticed that the knockdown of FoxO1 would reverse the antiproliferation properties of actein, indicating that Akt/FoxO1 pathway plays an important role in actein-induced effect towards HNSCC [41]. FoxO1 is one of many transcription factors among the FoxO family, which regulated various biological activities in HNSCC, such as cancer cell invasion and proliferation [73].
Activated p53 via phosphorylation act as a tumour suppressive molecule, which had been associated with induced apoptosis in HNSCC [74,75]. Moreover, it was previously reported that about half of HNSCC cases showed loss-of-function p53 gene mutation [76], making p53 an interesting target in improving efficiency of HNSCC therapy through reactivation of p53. Interestingly, it was discovered that wild-type p53 was functionally activated, but was downregulated by E6 oncoprotein in HPV-positive HNSCC, leading to tumorigenesis, while p53 was inactivated in HPV-negative HNSCC [76]. Nevertheless, neferine and ilimaquinone had shown via preclinical studies in HNSCC to upregulate or activate p53, leading to apoptosis [46,64]. However, further preclinical model of natural products with HPV-positive HNSCC cell lines may be required to understand the efficiency of respective natural product.
2.4. LC3-Dependent Autophagy
Autophagy is a major process involving protein degradation followed by turnover of cellular components, which helped maintain the intercellular homeostasis [77]. Light chain 3 (LC3) had been strongly associated with the regulation of autophagosome, a double membrane vesicle formed during autophagy [77,78]. During formation of autophagosome, cytosolic LC3 (LC3-I) is converted into the activated form of intra-autophagosomal LC3 (LC3-II) via uibiquitylation-like reaction catalysed by Atg7 and Atg3, by conjugating with phosphatidylethanolamine [77,78,79]. Therefore, the increased conversion of LC3-I to LC3-II indicates higher levels of autophagosome, and thus activation of autophagy, leading to tumour suppression [80]. The accumulation of p62 had also been reported to associate with induction of autophagy in HNSCC [81]. Honokiol, ilimaquinone, and nimbolide had been reported to increase LC3-II/LC3-I ratio with HNSCC preclinical models [42,45,47].
However, autophagy has been described as paradoxical as the it may also lead to tumour survival when certain condition is met [82,83,84,85]. Hypoxia, a condition where oxygen level is below physiological level, is a common feature in tumour progression when the oxygen supply could not met the demand due to the exponential growth of tumour [83,86]. Hypoxia-induced autophagy in tumour has been observed to induce tumour survival in vitro [83]. The knockdown of essential autophagy protein with xenograft model has also shown that inhibition of autophagy promote tumour suppression [84]. The turnover of cellular components by autophagy may essentially help with hypoxia and nutrient stress faced by exponentially growing tumour, thus enhancing tumour survival [83,84]. Further investigation on the relationship between autophagy and tumour progression or suppression is required to aid future clinical settings.
2.5. Bcl-2/Bax Signaling
Bcl-2 and Bax are proteins found in the mitochondrial membrane, nuclear envelop and endoplasmic reticulum [87,88]. Bcl-2 protein function to inhibit release of cytochrome c, and thus inhibiting intrinsic apoptosis, while Bax protein reverse the reaction, leading to induction of intrinsic apoptosis in HNSCC [89]. Cytochrome c when released would followed by a cascade reaction involving apoptotic protease activating factor 1 (Apaf-1), Caspase-9, Caspase-7 and Caspase-3, leading to apoptosis [89,90]. Preclinical studies in HNSCC with actein, xanthohumol, fucoidan, ilimaquinone, nimbolide, 4-O-methylhonokiol, and cinnamaldehyde had indicated higher Bax/Bcl-2 ratio (upregulating Bax and/or downregulating Bcl-2) on mitochondrial membrane, leading to induction of intrinsic apoptosis of HNSCC. [41,44,46,47,48,49,91]. The activation of Caspase-8 had also been associated with the activation of Caspase-7 and Caspase-3 followed by apoptosis induction, while Survivin act as an anti-apoptotic protein [75,92,93]. Upregulation of Caspase-8 in preclinical studies in HNSCC had been reported with ilimaquinone, while downregulation of Survivin had been observed with actein, xanthohumol, ilimaquinone, and Seco-A-ring oleanane [41,44,46,50].
2.6. Cell Cycle Arrest by Cyclin and CDK Signaling Pathway
The cell cycle act as a fundamental process for cancer progression in HNSCC, which is closely related to cell proliferation and differentiation [94]. It is well established that cyclins and cyclin-dependent kinases (Cdks) play an important role in regulation and transition of cell cycles [95,96,97]. G0/G1 transitioning is strongly dependent on cyclin-D/Cdk4/6 complexes, S phase entry is dependent on cyclin E/Cdk2 complex, S/G2 transitioning is dependent on cyclin A/Cdk2 complex, followed by mitotic phase entry which is dependent on cyclin A/Cdk1 complex, and finally M/G0 transitioning which is dependent on cyclin B/Cdk1 complex [98,99,100,101,102]. The inhibition of cell cycle transitioning would lead to cell cycle arrest and eventually cell death, which could be done via inhibiting cyclin-Cdk complexes formation in HNSCC [103]. Anti-mitogenic signals such as p16, p21 and p53 could inhibit cell cycle transition effectively by inhibiting cyclin-Cdk complexes formation, thus acting as an important target for cell cycle arrest induction [98,104,105]. Xanthohumol, neferine, fucoidan, hydroxygenkwanin, ilimaquinone, honokiol, trichodermin, and cinnamaldehyde had been discovered to induce cell cycle arrest via cyclin-dependent signaling with HNSCC preclinical models [44,45,46,48,49,56,64,106].
2.7. Potential Natural Products as Therapeutic Agent for HNSCC
Preclinical studies using HNSCC models with actein, salicylate, tanshinone IIA, xanthohumol, honokiol, trichodermin, psorachromene, and protocatechuic acid had shown promising molecular mechanisms against HNSCC cell lines and impaired tumor growth on xenografted animal model [24,41,42,43,44,45,56,65], among which actein, salicylate, xanthohumol, trichodermin, psorachromene, and protocatechuic acid are novel natural products yet to be reviewed by Aggarwal et al. [12]. Actein and xanthohumol are both pro-apoptosis phytochemicals which trigger apoptosis via Bax upregulation and/or Bcl-2 downregulation, while actein had also shown promising tumor suppressing activity via inhibition of Akt/FoxO1 signaling pathway [41,44]. Honokiol had also shown to interact with the MAPK pathway leading to inhibition of cell proliferation, making it a promising phytochemical for future clinical research [45].
Other natural products such as fucoidan, ilimaquinone, nimbolide, cinnamaldehyde, [(3β)-3-hydroxy-lup-20(29)-en-28-oic acid], Seco-A-ring oleanane, 4-O-methylhonokiol, sodium Danshensu, conocarpan, and hydroxygenkwanin had shown promising anti-cancer activity via inhibition of Akt-related and/or Caspase-related signaling pathways in HNSCC preclinical studies [46,47,48,49,50,55,91,106,107]. However, in vivo xenograft models were not carried out by the above natural products. Therefore, the anti-cancer activity of above natural products could be further investigated for the safety and efficacy outcome before moving forward to clinical trials. Table 1 summarizes in vitro preclinical studies using natural products on various HNSCC cell lines while Table 2 summarizes in vitro and in vivo preclinical studies of natural products.
3. Chemoprevention Properties of Natural Products Against HNSCC Oral Carcinogenesis Mechanism
Oral carcinogenesis often involved formation of abnormalities in the oral tissue known as oral potentially malignant disorder (OPMD) before proceeding to oral squamous cell carcinoma (OSCC), a major type of HNSCC [109,110]. Previous analysis had proposed an average malignant transformation (MT) rate of OPMD at 7.9%, while high risk OPMD such as erythroplakia had shown average MT rate of 33.1% [111]. However, to date, no preventive strategies including the use of drugs and/or surgical procedures is considered the standard of care for OPMDs, thus indicating the need to investigate the chemoprevention properties of various natural products against the oral carcinogenesis mechanism [112]. Several drugs such as celecoxib, erlotinib and metformin have been investigated for their HNSCC prevention properties via clinical trials on oral premalignant lesions [113,114,115]. However, the use of erlotinib and celecoxib has shown no significant result in reducing oral cancer-free survival rate while possessing higher toxicity [113,114], and the use of metformin has shown low clinical response rate (17%) in terms of reduction in lesion size [115].
In vivo studies involving the use of genetically altered rodents or rodents treated with chemical carcinogens could lead to site specific carcinogenesis, mimicking carcinogenesis in humans [116]. 4-nitroquinoline 1-oxide (4NQO) acts as a tobacco-mimicking carcinogen which had been widely used in carcinogen-induced HNSCC animal model, mainly due to the similarity in terms of genetic alteration and expression between 4NQO-induced mouse model and human oral carcinogenesis [117,118]. Other carcinogens including 7,12-dimethylbenz(a)anthracene (DMBA) and dibenzo[a,l]pyrene (DBP) had also been widely used as HNSCC inducing agent in animal models [64,119].
To date, several review studies had introduced various natural products as chemoprevention agents against HNSCC, where vitamin A, green tea extracts and curcumin had shown promising results in preclinical and clinical trials [29,30]. However, vitamin A has shown toxicity [31,32] while green tea extracts and curcumin [33,34] have both shown limitation in bioavailability. In a randomized chemoprevention trial reported by Papadimitrakopoulou et al. [32], low-dose 13-cis retinoic acid (a derivative of vitamin A) has induced grade 1 (45%), 2 (37%), 3 (15%) and 4 (1%) toxicity, majority in terms of cheilitis, conjunctivitis and skin reactions. Also, treatment of 2 out of 83 patients were halted due to toxicity [32]. Similarly, a phase II chemoprevention trial by Shin et al. [31] with combinations of interferon-alpha, 13-cis retinoic acid and alpha-tocopherol had induced mild to moderate non-hematologic toxicity. One patient was reported with severe throat infection due to beta-hemolytic streptococci which required emergency tracheostomy. However, the patient was able to complete the planned treatment after fully recovered from the infection [31]. Although severe toxicity or side effects due to 13-cis retinoic acid is rare, but it should not be neglected. Green tea extract on the other hand was reported to induce adverse effects such as insomnia, nausea, nervousness and headache, which most likely due to presence of caffeine in green tea extract [33]. The poor oral absorption of epigallocatechin-3-gallate, most abundant polyphenol in green tea extract, was reported by Tsao et al. [33], leading to variability in plasma epigallocatechin-3-gallate concentration. Similarly, phase I chemoprevention trial by Cheng et al. [34] with curcumin has also indicated the poor gastrointestinal absorption of curcumin as the peak serum curcumin concentration was recorded at 1.77µM with 8000 mg daily dosage. The poor bioavailability of both green tea extract and curcumin have introduced difficulties in dosage estimation as absorption varies among patients, which may lead to ineffective treatment. Therefore, in this review, we seek to provide more variety of promising natural products for their chemoprevention properties using 4NQO, DMBA or DBP induced carcinogenesis with animal models [25,64,119]. Table 3 summarized preclinical studies with natural products involved in chemoprevention of HNSCC investigated in the past 5 years.
Preclinical in vivo studies involving neferine and nimbolide had indicated inhibition in carcinogenesis and reduction in tumor growth of DMBA-induced HNSCC oral carcinogenesis, making both possible chemoprevention agents [41]. Similarly, calcitriol had successfully inhibited 4NQO-induced HNSCC oral carcinogenesis [25]. Interestingly, study by Vincent-Chong et al. [25] with calcitriol on 4NQO-induced carcinogenesis had shown the influence of stage of treatment intervention and duration of exposure to treatment to carcinogenesis, making it prominent to understand the pathway involved in during carcinogenesis and targeting the pathways with respective treatments for an effective intervention.
Calcitriol, nimbolide, and neferine are the three natural products being investigated in the preclinical studies for chemoprevention of carcinogen induced HNSCC oral carcinogenesis, which had also shown promising result by reducing or inhibiting induced-carcinogenesis, by inhibiting carcinogenesis-related molecular mechanisms [25,47,64].
4. Limitation and Future Direction
As multiple signaling pathways and cross-talk between pathways took place during carcinogenesis, and most recurrent or metastatic HNSCC had failed the primary standard of care, an effective treatment for cancer may require combined therapeutic approach such as the use of multiple signaling inhibitors combined with DNA damaging drugs for the most efficient outcome [26]. Therefore, the synergic or antagonistic effects of natural products with the standard of care such as chemotherapy (cisplatin, cetuximab, pembrolizumab and nivolumab) and radiotherapy should be analysed using preclinical models. However, less than 10% of studies reviewed had reported the combination effects of natural products to the standard of care. For instance, xanthohumol, psorachromene, honokiol, calcitriol and salicylate had been shown to provide synergistic effects with standard of care treatment such as chemotherapy and radiotherapy using HNSCC preclinical models [24,25,42,44,45].
As mentioned, the major reason for low survival rate of HNSCC was due to late diagnosis and risk factors associated with HNSCC progression, which would then lead to risk of recurrent or metastatic SCC [5,12]. Chemoprevention therapy could potentially act as an important barrier to lower the risk of recurrent or metastatic SCC, and malignant transformation of OPMD, and therefore should be widely studied in the future. Previous prevention of HNSCC involving single agent chemotherapy such as retinoids and isotretinoin possesses high toxicity and low efficacy, indicating the need for development of new chemoprevention agents either as alternative or adjunctive agents for HNSCC prevention. In the current review, only 5 out of 37 studies had explored the potential usage of natural products as chemoprevention therapy using 4NQO/DMBA/DBP-induced oral carcinogenesis [25,47,64,119]. Furthermore, the stage and duration of intervention of natural products on oral carcinogenesis should also be extensively explored as it has been proposed by Vincent-Chong et al. [25] to strongly associate with the progression of carcinogenesis. All 6 natural products (Table 3) are strongly encouraged to proceed with clinical trials for high-risk OPMD patients as previous trials with celecoxib, erlotinib and metformin with mild to advanced OPMD had shown no significant clinical improvements [113,114,115].
Study reported by Dai et al. [120] using various cancer (melanoma, breast, colon and liver cancer cell lines (H1299, BT549, MDA-MB-231, MDA-MB-468, SW620, MHCC97H and B16F10) in a preclinical study had indicated the crucial role of immunomodulatory roles of natural products in anti-cancer treatments, with the involvement of CD3+ CD8+ T lymphocytes in a co-culture system together with cancer cell lines. Similarly, Cattanaeo et al. [121] and Neal et al. [122] have proposed the use of co-culture organoid-tumor reactive T lymphocyte system to investigate the role of anti-PD-1 drugs on T lymphocytes activity, which has been employed by other studies to screen natural product derived drugs and epigenetic inhibitors for the non-cytotoxic T lymphocytes immunomodulating effects [120,123]. Apart from in vitro co-culture system proposed, Verma et al. [124] had implemented in vivo RPMOC1 synergic HNSCC animal model to investigate the effects of non-oncological drug, calcitriol, on immunomodulatory of T lymphocytes, which is also the only preclinical study involving synergic HNSCC in vivo model present at the time of preparing this review. To address this paucity, studies using in vitro (immune-tumor co-culture system) and in vivo synergic HNSCC models are urgently needed to investigate the role of natural products listed in Table 1, 2 and 3 to enhance the anti-tumor effect of immune checkpoint inhibitors.
Among the reviewed natural products, actein, salicylate, tanshinone IIA, xanthohumol, honokiol, trichodermin, psorachromene and protocatechuic acid had been investigated as chemotherapeutic agents with HNSCC cell lines and xenografted animal models, and shown promising targeted molecular mechanisms against HNSCC, making them an ideal candidate for further clinical trials for safety and efficacy analysis [24,41,42,43,44,45,56,65]. Furthermore, salicylate and xanthohumol had been investigated to provide synergistic effect with cisplatin and radiotherapy respectively, making them the strongest candidate for future clinical trials for HNSCC or OPMD patients [42,44]. Finally, calcitriol, nimbolide and neferine are the major natural products being investigated from this review, which had shown promising chemoprevention properties against induced-carcinogenesis animal models, and are encouraged to further investigate the safety and efficacy with human trials, against high risk OPMD such as erythroplakia and leukoplakia patients via clinical trials [25,47,64].
5. Conclusions
Both chemotherapeutic and chemoprevention approach had shown promising anti-cancer effects through preclinical studies involving various HNSCC cell lines and animal models via various pathway (Figure 2). However, the lack of extensive molecular mechanisms of natural products and lack of combination of natural products with the current standard of care had limited the progression of natural products as a new chemotherapeutic drug. Apart from that, only one study of using calcitriol to determine the immunomodulatory effect of natural product in the context of HNSCC, highlighted the effort to investigate the role of these natural products for their immunomodulatory effect as immune checkpoint inhibitor has been recognized as one of the standard of care for HNSCC patient. The low intrinsic toxicity of natural products in normal cells and significant therapeutic effects toward cancer cells had spark the interest in oncology studies in the recent years. The synergistic effect of natural products with standard of care including chemotherapy, radiotherapy and immunotherapy had showed promising properties of natural products as both the alternative and adjunctive chemotherapeutic or chemoprevention agents in cancer treatment or prevention respectively.
As a conclusion, we attempted to provide a comprehensive database of natural products used for in vivo and in vitro preclinical studies involving HNSCC in the recent years, which would ease researchers in the near future in identifying effective natural products which have shown promising chemotherapeutic and chemoprevention properties against HNSCC while possessing low toxicity. Natural products have shown to be promising molecular targets for therapeutic and prevention against HNSCC, making it a great alternative and adjunctive agents in cancer treatment. However, further toxicity profiles should be analysed for natural products in future clinical trials to ensure the safety and efficacy of natural products against HNSCC.
Author Contributions
Conceptualization, Vui King Vincent-Chong; Investigation, Yoon Xuan Liew, Lee Peng Karen-Ng, Vui King Vincent-Chong; Visualization, Yoon Xuan Liew; Writing – Original Draft Preparation, Yoon Xuan Liew; Writing – Review & Editing, Lee Peng Karen-Ng and Vui King Vincent-Chong.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and Neck Squamous Cell Carcinoma. Nat. Rev. Dis. Prim. 2020, 6. [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [CrossRef]
- Magnes, T.; Wagner, S.M.; Melchardt, T.; Weiss, L.; Rinnerthaler, G.; Huemer, F.; Kopp, M.; Gampenrieder, S.P.; Mayrbäurl, B.; Füreder, T.; et al. Postoperative Chemoradiotherapy with Cisplatin Is Superior to Radioimmunotherapy with Cetuximab and Radiotherapy Alone: Analysis of the Austrian Head and Neck Cancer Registry of the AGMT. Wien. Klin. Wochenschr. 2021, 133, 1131–1136. [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA. Cancer J. Clin. 2022, 72, 7–33. [CrossRef]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Cohen, R.B.; Jones, C.U.; Sur, R.K.; Raben, D.; Baselga, J.; Spencer, S.A.; Zhu, J.; et al. Radiotherapy plus Cetuximab for Locoregionally Advanced Head and Neck Cancer: 5-Year Survival Data from a Phase 3 Randomised Trial, and Relation between Cetuximab-Induced Rash and Survival. Lancet Oncol. 2010, 11, 21–28. [CrossRef]
- Ferris, R.L.; Blumenschein, G.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [CrossRef]
- Talamini, R.; Bosetti, C.; La Vecchia, C.; Dal Maso, L.; Levi, F.; Bidoli, E.; Negri, E.; Pasche, C.; Vaccarella, S.; Barzan, L.; et al. Combined Effect of Tobacco and Alcohol on Laryngeal Cancer Risk: A Case-Control Study. Cancer Causes Control 2002, 13, 957–964. [CrossRef]
- Lewin, F.; Norell, S.E.; Johansson, H.; Gustavsson, P.; Wennerberg, J.; Biörklund, A.; Rutqvist, L.E. Smoking Tobacco, Oral Snuff, and Alcohol in the Etiology of Squamous Cell Carcinoma of the Head and Neck A Population-Based Case-Referent Study in Sweden. Cancer 1998, 82, 1367–1375. [CrossRef]
- Hansson, B.G.; Rosenquist, K.; Antonsson, A.; Wennerberg, J.; Schildt, E.B.; Bladström, A.; Andersson, G. Strong Association between Infection with Human Papillomavirus and Oral and Oropharyngeal Squamous Cell Carcinoma: A Population-Based Case-Control Study in Southern Sweden. Acta Otolaryngol. 2005, 125, 1337–1344. [CrossRef]
- Van Wyk, C.W.; Stander, I.; Padayachee, A.; Grobler-Rabie, A.F. The Areca Nut Chewing Habit and Oral Squamous Cell Carcinoma in South African Indians. A Retrospective Study. South African Med. J. 1993, 83, 425–429.
- Haddad, R.I.; Shin, D.M. Recent Advances in Head and Neck Cancer Reconstruction. N. Engl. J. Med. 2008, 359, 1143–1154. [CrossRef]
- Aggarwal, N.; Yadav, J.; Chhakara, S.; Janjua, D.; Tripathi, T.; Chaudhary, A.; Chhokar, A.; Thakur, K.; Singh, T.; Bharti, A.C. Phytochemicals as Potential Chemopreventive and Chemotherapeutic Agents for Emerging Human Papillomavirus–Driven Head and Neck Cancer: Current Evidence and Future Prospects. Front. Pharmacol. 2021, 12. [CrossRef]
- Posner, M.R.; Hershock, D.M.; Blajman, C.R.; Mickiewicz, E.; Winquist, E.; Gorbounova, V.; Tjulandin, S.; Shin, D.M.; Cullen, K.; Ervin, T.J.; et al. Cisplatin and Fluorouracil Alone or with Docetaxel in Head and Neck Cancer Marshall. N. Engl. J. Med. 2007, 357, 1705–1715. [CrossRef]
- Hashim, D.; Genden, E.; Posner, M.; Hashibe, M.; Boffetta, P. Head and Neck Cancer Prevention: From Primary Prevention to Impact of Clinicians on Reducing Burden. Ann. Oncol. 2019, 30, 744–756. [CrossRef]
- Brockstein, B.; Haraf, D.J.; Rademaker, A.W.; Kies, M.S.; Stenson, K.M.; Rosen, F.; Mittal, B.B.; Pelzer, H.; Fung, B.B.; Witt, M.E.; et al. Patterns of Failure, Prognostic Factors and Survival in Locoregionally Advanced Head and Neck Cancer Treated with Concomitant Chemoradiotherapy: A 9-Year, 337-Patient, Multi-Institutional Experience. Ann. Oncol. 2004, 15, 1179–1186. [CrossRef]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.B.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J.; et al. Results of Radiotherapy plus Cetuximab for Squamous Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2006, 354, 567–578. [CrossRef]
- Naruse, T.; Yanamoto, S.; Matsushita, Y.; Sakamoto, Y.; Morishita, K.; Ohba, S.; Shiraishi, T.; Yamada, S.-I.; Asahina, I.; Umeda, M. Cetuximab for the Treatment of Locally Advanced and Recurrent/Metastatic Oral Cancer: An Investigation of Distant Metastasis. Mol. Clin. Oncol. 2016, 5, 246–252. [CrossRef]
- Sok, J.C.; Coppelli, F.M.; Thomas, S.M.; Lango, M.N.; Xi, S.; Hunt, J.L.; Freilino, M.L.; Graner, M.W.; Wikstrand, C.J.; Bigner, D.D.; et al. Mutant Epidermal Growth Factor Receptor (EGFRvIII) Contributes to Head and Neck Cancer Growth and Resistance to EGFR Targeting. Clin. Cancer Res. 2006, 12, 5064–5073. [CrossRef]
- US Food and Drug Administration FDA Approves Pembrolizumab for First-Line Treatment of Head and Neck Squamous Cell Carcinoma. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-first-line-treatment-head-and-neck-squamous-cell-carcinoma.
- US Food and Drug Administration Nivolumab for SCCHN. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/nivolumab-scchn.
- dos Santos, L. V.; Abrahão, C.M.; William, W.N. Overcoming Resistance to Immune Checkpoint Inhibitors in Head and Neck Squamous Cell Carcinomas. Front. Oncol. 2021, 11, 1–16. [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [CrossRef]
- National Cancer Institute Dictionary of Cancer Terms. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms (accessed on 26 September 2022).
- Wang, T.H.; Leu, Y.L.; Chen, C.C.; Shieh, T.M.; Lian, J.H.; Chen, C.Y. Psorachromene Suppresses Oral Squamous Cell Carcinoma Progression by Inhibiting Long Non-Coding RNA GAS5 Mediated Epithelial-Mesenchymal Transition. Front. Oncol. 2019, 9, 1–12. [CrossRef]
- Vincent-Chong, V.K.; DeJong, H.; Attwood, K.; Hershberger, P.A.; Seshadri, M. Preclinical Prevention Trial of Calcitriol: Impact of Stage of Intervention and Duration of Treatment on Oral Carcinogenesis. Neoplasia (United States) 2019, 21, 376–388. [CrossRef]
- Hashem, S.; Ali, T.A.; Akhtar, S.; Nisar, S.; Sageena, G.; Ali, S.; Al-Mannai, S.; Therachiyil, L.; Mir, R.; Elfaki, I.; et al. Targeting Cancer Signaling Pathways by Natural Products: Exploring Promising Anti-Cancer Agents. Biomed. Pharmacother. 2022, 150, 113054. [CrossRef]
- Sever, R.; Brugge, J.S. Signal Transduction in Cancer. Cold Spring Harb. Perspect. Med. 2015, 5. [CrossRef]
- Sanchez-Vega, F.; Mina, M.; Armenia Pathways, Oncogenic Signaling Cancer, The Atlas, Genome. 2019, 173, 321–337. [CrossRef]
- Crooker, K.; Aliani, R.; Ananth, M.; Arnold, L.; Anant, S.; Thomas, S.M. A Review of Promising Natural Chemopreventive Agents for Head and Neck Cancer; 2018; Vol. 11; ISBN 1913588467.
- Rahman, M.A.; Amin, A.R.M.R.; Shin, D.M. Chemopreventive Potential of Natural Compounds in Head and Neck Cancer. Nutr. Cancer 2010, 62, 973–987. [CrossRef]
- Shin, D.M.; Khuri, F.R.; Murphy, B.; Garden, A.S.; Clayman, G.; Francisco, M.; Liu, D.; Glisson, B.S.; Ginsberg, L.; Papadimitrakopoulou, V.; et al. Combined Interferon-Alfa, 13-Cis-Retinoic Acid, and Alpha-Tocopherol in Locally Advanced Head and Neck Squamous Cell Carcinoma: Novel Bioadjuvant Phase II Trial. J. Clin. Oncol. 2001, 19, 3010–3017. [CrossRef]
- Papadimitrakopoulou, V.A.; Lee, J.J.; William, W.N.; Martin, J.W.; Thomas, M.; Kim, E.S.; Khuri, F.R.; Shin, D.M.; Feng, L.; Waun, K.H.; et al. Randomized Trial of 13-Cis Retinoic Acid Compared with Retinyl Palmitate with or without Beta-Carotene in Oral Premalignancy. J. Clin. Oncol. 2009, 27, 599–604. [CrossRef]
- Tsao, A.S.; Liu, D.; Martin, J.; Tang, X.M.; Lee, J.J.; El-Naggar, A.K.; Wistuba, I.; Culotta, K.S.; Mao, L.; Gillenwater, A.; et al. Phase II Randomized, Placebo-Controlled Trial of Green Tea Extract in Patients with High-Risk Oral Premalignant Lesions. Cancer Prev. Res. 2009, 2, 931–941. [CrossRef]
- Cheng, A.L.; Hsu, C.H.; Lin, J.K.; Hse, M.W.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Wu, M.S.; et al. Phase I Clinical Trial of Curcumin, a Chemopreventive Agent, in Patients with High-Risk or Pre-Malignant Lesions. Anticancer Res. 2001, 21, 2895–2900.
- Molinolo, A.A.; Hewitt, S.M.; Amornphimoltham, P.; Keelawat, S.; Rangdaeng, S.; García, A.M.; Raimondi, A.R.; Jufe, R.; Itoiz, M.; Gao, Y.; et al. Dissecting the Akt/Mammalian Target of Rapamycin Signaling Network: Emerging Results from the Head and Neck Cancer Tissue Array Initiative. Clin. Cancer Res. 2007, 13, 4964–4973. [CrossRef]
- Freudlsperger, C.; Horn, D.; Weißfuß, S.; Weichert, W.; Weber, K.J.; Saure, D.; Sharma, S.; Dyckhoff, G.; Grabe, N.; Plinkert, P.; et al. Phosphorylation of AKT(Ser473) Serves as an Independent Prognostic Marker for Radiosensitivity in Advanced Head and Neck Squamous Cell Carcinoma. Int. J. Cancer 2015, 136, 2775–2785. [CrossRef]
- Zumsteg, Z.S.; Morse, N.; Krigsfeld, G.; Gupta, G.; Higginson, D.S.; Lee, N.Y.; Morris, L.; Ganly, I.; Shiao, S.L.; Powell, S.N.; et al. Taselisib (GDC-0032), a Potent β-Sparing Small Molecule Inhibitor of PI3K, Radiosensitizes Head and Neck Squamous Carcinomas Containing Activating PIK3CA Alterations. Clin. Cancer Res. 2016, 22, 2009–2019. [CrossRef]
- Iwase, M.; Yoshiba, S.; Uchid, M.; Takaoka, S.; Kurihara, Y.; Ito, D.; Hatori, M.; Shintani, S. Enhanced Susceptibility to Apoptosis of Oral Squamous Cell Carcinoma Cells Subjected to Combined Treatment with Anticancer Drugs and Phosphatidylinositol 3-Kinase Inhibitors. Int. J. Oncol. 2007, 31, 1141–1147.
- Cantley, L.C. The Phosphoinositide 3-Kinase Pathway. Science (80-. ). 2002, 296, 1655–1657. [CrossRef]
- Sarbassov, D.D.; Guertin, D.A.; Ali, S.M.; Sabatini, D.M. Phosphorylation and Regulation of Akt/PKB by the Rictor-MTOR Complex. Science (80-. ). 2005, 307, 1098–1101. [CrossRef]
- Zhao, C.; Zhang, Z.; Dai, X.; Wang, J.; Liu, H.; Ma, H. Actein Antagonizes Oral Squamous Cell Carcinoma Proliferation through Activating FoxO1. Pharmacology 2021, 106, 551–563. [CrossRef]
- Zhang, X.; Wang, F.; Zeng, Y.; Zhu, X.; Peng, L.; Zhang, L.; Gu, J.; Han, H.; Yi, X.; Shi, J. Salicylate Sensitizes Oral Squamous Cell Carcinoma to Chemotherapy through Targeting MTOR Pathway. Oral Dis. 2020, 26, 1131–1140. [CrossRef]
- Li, M.; Gao, F.; Zhao, Q.; Zuo, H.; Liu, W.; Li, W. Tanshinone IIA Inhibits Oral Squamous Cell Carcinoma via Reducing Akt-c-Myc Signaling-Mediated Aerobic Glycolysis. Cell Death Dis. 2020, 11. [CrossRef]
- Li, M.; Gao, F.; Yu, X.; Zhao, Q.; Zhou, L.; Liu, W.; Li, W. Promotion of Ubiquitination-Dependent Survivin Destruction Contributes to Xanthohumol-Mediated Tumor Suppression and Overcomes Radioresistance in Human Oral Squamous Cell Carcinoma. J. Exp. Clin. Cancer Res. 2020, 39, 1–15. [CrossRef]
- Huang, K.J.; Kuo, C.H.; Chen, S.H.; Lin, C.Y.; Lee, Y.R. Honokiol Inhibits in Vitro and in Vivo Growth of Oral Squamous Cell Carcinoma through Induction of Apoptosis, Cell Cycle Arrest and Autophagy. J. Cell. Mol. Med. 2018, 22, 1894–1908. [CrossRef]
- Lin, C.W.; Bai, L.Y.; Su, J.H.; Chiu, C.F.; Lin, W.Y.; Huang, W.T.; Shih, M.C.; Huang, Y.T.; Hu, J.L.; Weng, J.R. Ilimaquinone Induces Apoptosis and Autophagy in Human Oral Squamous Cell Carcinoma Cells. Biomedicines 2020, 8, 1–11. [CrossRef]
- Sophia, J.; Kowshik, J.; Dwivedi, A.; Bhutia, S.K.; Manavathi, B.; Mishra, R.; Nagini, S. Nimbolide, a Neem Limonoid Inhibits Cytoprotective Autophagy to Activate Apoptosis via Modulation of the PI3K/Akt/GSK-3β Signalling Pathway in Oral Cancer. Cell Death Dis. 2018, 9. [CrossRef]
- Aggarwal, S.; Bhadana, K.; Singh, B.; Rawat, M.; Mohammad, T.; Al-Keridis, L.A.; Alshammari, N.; Hassan, M.I.; Das, S.N. Cinnamomum Zeylanicum Extract and Its Bioactive Component Cinnamaldehyde Show Anti-Tumor Effects via Inhibition of Multiple Cellular Pathways. Front. Pharmacol. 2022, 13, 1–14. [CrossRef]
- Zhang, N.; Gao, L.; Ren, W.; Li, S.; Zhang, D.; Song, X.; Zhao, C.; Zhi, K. Fucoidan Affects Oral Squamous Cell Carcinoma Cell Functions in Vitro by Regulating FLNA-Derived Circular RNA. Ann. N. Y. Acad. Sci. 2020, 1462, 65–78. [CrossRef]
- Aswathy, M.; Banik, K.; Parama, D.; Sasikumar, P.; Harsha, C.; Joseph, A.G.; Sherin, D.R.; Thanathu, M.K.; Kunnumakkara, A.B.; Vasu, R.K. Exploring the Cytotoxic Effects of the Extracts and Bioactive Triterpenoids from Dillenia Indica against Oral Squamous Cell Carcinoma: A Scientific Interpretation and Validation of Indigenous Knowledge. ACS Pharmacol. Transl. Sci. 2021, 4, 834–847. [CrossRef]
- Schulz, L.; Pries, R.; Lanka, A.S.; Drenckhan, M.; Rades, D.; Wollenberg, B. Inhibition of GSK3α/β Impairs the Progression of HNSCC. Oncotarget 2018, 9, 27630–27644. [CrossRef]
- Ugolkov, A. V.; Matsangou, M.; Taxter, T.J.; O’halloran, T. V.; Cryns, V.L.; Giles, F.J.; Mazar, A.P. Aberrant Expression of Glycogen Synthase Kinase-3β in Human Breast and Head and Neck Cancer. Oncol. Lett. 2018, 16, 6437–6444. [CrossRef]
- Marconi, G.D.; Della Rocca, Y.; Fonticoli, L.; Melfi, F.; Rajan, T.S.; Carradori, S.; Pizzicannella, J.; Trubiani, O.; Diomede, F. C-Myc Expression in Oral Squamous Cell Carcinoma: Molecular Mechanisms in Cell Survival and Cancer Progression. Pharmaceuticals 2022, 15, 1–14. [CrossRef]
- Bancroft, C.C.; Chen, Z.; Dong, G.; Sunwoo, J.B.; Yeh, N.; Park, C.; Van Waes, C. Coexpression of Proangiogenic Factors IL-8 and VEGF by Human Head and Neck Squamous Cell Carcinoma Involves Coactivation by MEK-MAPK and IKK-NF-ΚB Signal Pathways. Clin. Cancer Res. 2001, 7, 435–442.
- Kumar, V.B.; Lin, S.H.; Mahalakshmi, B.; Lo, Y.S.; Lin, C.C.; Chuang, Y.C.; Hsieh, M.J.; Chen, M.K. Sodium Danshensu Inhibits Oral Cancer Cell Migration and Invasion by Modulating P38 Signaling Pathway. Front. Endocrinol. (Lausanne). 2020, 11, 1–10. [CrossRef]
- Li, Z.; Cao, L.; Yang, C.; Liu, T.; Zhao, H.; Luo, X.; Chen, Q. Protocatechuic Acid-Based Supramolecular Hydrogel Targets SerpinB9 to Achieve Local Chemotherapy for OSCC. 2022. [CrossRef]
- Gkouveris, I.; Nikitakis, N.; Karanikou, M.; Rassidakis, G.; Sklavounou, A. JNK1/2 Expression and Modulation of STAT3 Signaling in Oral Cancer. Oncol. Lett. 2016, 12, 699–706. [CrossRef]
- O-charoenrat, P.; Rhys-Evans, P.H.; Eccles, S.A. Expression of Matrix Metalloproteinases and Their Inhibitors Correlates With Invasion and Metastasis in Squamous Cell Carcinoma of the Head and Neck. Arch Otolaryngol Head Neck Surg 2001, 127, 813–820.
- Carmeliet, P. VEGF as a Key Mediator of Angiogenesis in Cancer. Oncology 2005, 69, 4–10. [CrossRef]
- Pepper, M.S.; Ferrara, N.; Orci, L.; Montesano, R. Potent Synergism between Vascular Endothelial Growth Factor and Basic Fibroblast Growth Factor in the Induction of Angiogenesis in Vitro. Biochem. Biophys. Res. Commun. 1992, 189, 824–831.
- Zhang, Y.H.; Wei, W.; Xu, H.; Wang, Y.Y.; Wu, W.X. Inducing Effects of Hepatocyte Growth Factor on the Expression of Vascular Endothelial Growth Factor in Human Colorectal Carcinoma Cells through MEK and PI3K Signaling Pathways. Chin. Med. J. (Engl). 2007, 120, 743–748. [CrossRef]
- Lun, M.; Zhang, P.L.; Pellitteri, P.K.; Law, A.; Kennedy, T.L.; Brown, R.E. Nuclear Factor-KappaB Pathway as a Therapeutic Target in Head and Neck Squamous Cell Carcinoma: Pharmaceutical and Molecular Validation in Human Cell Lines Using Velcade and SiRNA/NF-ΚB. Ann. Clin. Lab. Sci. 2005, 35, 251–258.
- Oeckinghaus, A.; Ghosh, S. The NF-ΚB Family of Transcription Factors and Its Regulation. Cold Spring Harb. Perspect. Biol. 2009, 1–15.
- Wang, J.; Hu, Y.; Yuan, J.; Zhang, Y.; Wang, Y.; Yang, Y.; Alahmadi, T.A.; Ali Alharbi, S.; Zhuang, Z.; Wu, F. Chemomodulatory Effect of Neferine on DMBA-Induced Squamous Cell Carcinogenesis: Biochemical and Molecular Approach. Environ. Toxicol. 2021, 36, 460–471. [CrossRef]
- Chen, H.; Lo, Y.; Lin, C.; Lee, T.; Leung, W.; Wang, S.; Lin, I.; Lin, M.; Lee, C. Biomedicine & Pharmacotherapy Trichodermin Inhibits the Growth of Oral Cancer through Apoptosis-Induced Mitochondrial Dysfunction and HDAC-2-Mediated Signaling. Biomed. Pharmacother. 2022, 153, 113351. [CrossRef]
- Ihle, J.N. The Stat Family in Cytokine Signaling. Curr. Opin. Cell Biol. 2001, 13, 211–217. [CrossRef]
- Sun, S.S.; Zhou, X.; Huang, Y.Y.; Kong, L.P.; Mei, M.; Guo, W.Y.; Zhao, M.H.; Ren, Y.; Shen, Q.; Zhang, L. Targeting STAT3/MiR-21 Axis Inhibits Epithelial-Mesenchymal Transition via Regulating CDK5 in Head and Neck Squamous Cell Carcinoma. Mol. Cancer 2015, 14, 1–11. [CrossRef]
- Zhou, X.; Ren, Y.; Liu, A.; Han, L.; Zhang, K.; Li, S.; Li, P.; Li, P.; Kang, C.; Wang, X.; et al. STAT3 Inhibitor WP1066 Attenuates MiRNA-21 to Suppress Human Oral Squamous Cell Carcinoma Growth in Vitro and in Vivo. Oncol. Rep. 2014, 31, 2173–2180. [CrossRef]
- Riebe, C.; Pries, R.; Schroeder, K.N.; Wollenberg, B. Phosphorylation of STAT3 in Head and Neck Cancer Requires P38 MAPKinase, Whereas Phosphorylation of STAT1 Occurs via a Different Signaling Pathway. Anticancer Res. 2011, 31, 3819–3825.
- Zhao, C.; Yang, L.; Zhou, F.; Yu, Y.; Du, X.; Xiang, Y.; Li, C.; Huang, X.; Xie, C.; Liu, Z.; et al. Feedback Activation of EGFR Is the Main Cause for STAT3 Inhibition-Irresponsiveness in Pancreatic Cancer Cells. Oncogene 2020, 39, 3997–4013. [CrossRef]
- Ning, Y.; Cui, Y.; Li, X.; Cao, X.; Chen, A.; Xu, C.; Cao, J.; Luo, X. Co-Culture of Ovarian Cancer Stem-like Cells with Macrophages Induced SKOV3 Cells Stemness via IL-8/STAT3 Signaling. Biomed. Pharmacother. 2018, 103, 262–271. [CrossRef]
- Yang, J.; Liao, D.; Chen, C.; Liu, Y.; Chuang, T.H.; Xiang, R.; Markowitz, D.; Reisfeld, R.A.; Luo, Y. Tumor-Associated Macrophages Regulate Murine Breast Cancer Stem Cells through a Novel Paracrine Egfr/Stat3/Sox-2 Signaling Pathway. Stem Cells 2013, 31, 248–258. [CrossRef]
- Chan, C.Y.; Huang, S.Y.; Sheu, J.J.C.; Roth, M.M.; Chou, I.T.; Lien, C.H.; Lee, M.F.; Huang, C.Y. Transcription Factor HBP1 Is a Direct Anti-Cancer Target of Transcription Factor FOXO1 in Invasive Oral Cancer. Oncotarget 2017, 8, 14537–14548. [CrossRef]
- Roh, J.L.; Kang, S.K.; Minn, I.; Califano, J.A.; Sidransky, D.; Koch, W.M. P53-Reactivating Small Molecules Induce Apoptosis and Enhance Chemotherapeutic Cytotoxicity in Head and Neck Squamous Cell Carcinoma. Oral Oncol. 2011, 47, 8–15. [CrossRef]
- Khan, Z.; Tiwari, R.P.; Mulherker, R.; Sah, N.K.; Prasad, G.B.; Shrivastava, B.R.; Bisen, P.S. Detection of Survivin and P53 in Human Oral Cancer: Correlation with Clinicopathological Findings. Head Neck 2009, 31, 1039–1048. [CrossRef]
- The Cancer Genome Atlas Network Comprehensive Genomic Characterization of Head and Neck Squamous Cell Carcinomas. Nature 2015, 517, 576–582. [CrossRef]
- Tanida, I.; Ueno, T.; Kominami, E. LC3 and Autophagy. Methods Mol. Biol. 2008, 445, 77–88. [CrossRef]
- Kabeya, Y.; Mizushima, N.; Ueno, T.; Yamamoto, A.; Kirisako, T.; Noda, T.; Kominami, E.; Ohsumi, Y.; Yoshimori, T. LC3, a Mammalian Homologue of Yeast Apg8p, Is Localized in Autophagosome Membranes after Processing. EMBO J. 2000, 19, 5720–5728. [CrossRef]
- Yun, C.W.; Lee, S.H. The Roles of Autophagy in Cancer. Int. J. Mol. Sci. 2018, 19, 1–18. [CrossRef]
- Khan, T.; Relitti, N.; Brindisi, M.; Magnano, S.; Zisterer, D.; Gemma, S.; Butini, S.; Campiani, G. Autophagy Modulators for the Treatment of Oral and Esophageal Squamous Cell Carcinomas. Med. Res. Rev. 2020, 40, 1002–1060. [CrossRef]
- Lee, M.; Nam, H.Y.; Kang, H.B.; Lee, W.H.; Lee, G.H.; Sung, G.J.; Han, M.W.; Cho, K.J.; Chang, E.J.; Choi, K.C.; et al. Epigenetic Regulation of P62/SQSTM1 Overcomes the Radioresistance of Head and Neck Cancer Cells via Autophagy-Dependent Senescence Induction. Cell Death Dis. 2021, 12. [CrossRef]
- Eisenberg-Lerner, A.; Kimchi, A. The Paradox of Autophagy and Its Implication in Cancer Etiology and Therapy. Cell D 2009, 14, 376–391. [CrossRef]
- Garcia-medina, R.; Gounon, P.; Chiche, J.; Pouysse, J.; Mazure, N.M. Hypoxia-Induced Autophagy Is Mediated through Hypoxia-Inducible Factor Induction of BNIP3 and BNIP3L via Their BH3 Domains †. 2009, 29, 2570–2581. [CrossRef]
- Wei, H.; Wei, S.; Gan, B.; Peng, X.; Zou, W.; Guan, J. Suppression of Autophagy by FIP200 Deletion Inhibits Mammary Tumorigenesis. 2011, 1510–1527. [CrossRef]
- Singh, S.S.; Vats, S.; Chia, A.Y.; Zea, T.; Shuo, T.; Mei, D.; Ong, S.; Arfuso, F.; Yap, C.T.; Cher, B.; et al. Dual Role of Autophagy in Hallmarks of Cancer. Oncogene 2018, 1142–1158. [CrossRef]
- Muz, B.; Azab, A.K. The Role of Hypoxia in Cancer Progression , Angiogenesis , Metastasis , and Resistance to Therapy. 2015, 83–92.
- Oltval, Z.N.; Milliman, C.L.; Korsmeyer, S.J. Bcl-2 Heterodimerizes in Vivo with a Conserved Homolog, Bax, That Accelerates Programed Cell Death. Cell 1993, 74, 609–619. [CrossRef]
- Hockenbery, D.; Nuñez, G.; Milliman, C.; Schreiber, R.D.; Korsmeyer, S.J. Bcl-2 Is an Inner Mitochondrial Membrane Protein That Blocks Programmed Cell Death. Nature 1990, 348, 334–336.
- Loro, L.L.; Vintermyr, O.K.; Liavaag, P.G.; Jonsson, R.; Johannessen, A.C. Oral Squamous Cell Carcinoma Is Associated with Decreased Bcl-2/Bax Expression Ratio and Increased Apoptosis. Hum. Pathol. 1999, 30, 1097–1105. [CrossRef]
- Zou, H.; Henzel, W.J.; Liu, X. Apaf-1, a Human Protein Homologous to C. Elegans CED-4, Participates In. Cell 1997, 90, 405–413.
- Xiao, S.; Chen, F.; Gao, C. Antitumor Activity of 4-O-Methylhonokiol in Human Oral Cancer Cells Is Mediated via ROS Generation, Disruption of Mitochondrial Potential, Cell Cycle Arrest and Modulation of Bcl-2/Bax Proteins. J. B.U.ON. 2017, 22, 1577–1581.
- Liu, J.; Uematsu, H.; Tsuchida, N.; Ikeda, M.A. Essential Role of Caspase-8 in P53/P73-Dependent Apoptosis Induced by Etoposide in Head and Neck Carcinoma Cells. Mol. Cancer 2011, 10, 1–13. [CrossRef]
- Liu, J.; Uematsu, H.; Tsuchida, N.; Ikeda, M.A. Association of Caspase-8 Mutation with Chemoresistance to Cisplatin in HOC313 Head and Neck Squamous Cell Carcinoma Cells. Biochem. Biophys. Res. Commun. 2009, 390, 989–994. [CrossRef]
- Coleman, S.C.; Stewart, Z.A.; Day, T.A.; Netterville, J.L.; Burkey, B.B.; Pietenpol, J.A. Analysis of Cell-Cycle Checkpoint Pathways in Head and Neck Cancer Cell Lines: Implications for Therapeutic Strategies. Arch. Otolaryngol. - Head Neck Surg. 2002, 128, 167–176. [CrossRef]
- Norbury, C.; Nurse, P. Animal Cell Cycles and Their Control. Annu. Rev. Biochem. 1992, 61, 441–470. [CrossRef]
- Xiong, Y.; Connolly, T.; Futcher, B.; Beach, D. Human D-Type Cyclin. Cell 1991, 65, 691–699. [CrossRef]
- Matsushime, H.; Ewen, M.E.; Strom, D.K.; Kato, J.Y.; Hanks, S.K.; Roussel, M.F.; Sherr, C.J. Identification and Properties of an Atypical Catalytic Subunit (P34PSK-J3/Cdk4) for Mammalian D Type G1 Cyclins. Cell 1992, 71, 323–334. [CrossRef]
- Lea, N.C.; Orr, S.J.; Stoeber, K.; Williams, G.H.; Lam, E.W.-F.; Ibrahim, M.A.A.; Mufti, G.J.; Thomas, N.S.B. Commitment Point during G 0 →G 1 That Controls Entry into the Cell Cycle . Mol. Cell. Biol. 2003, 23, 2351–2361. [CrossRef]
- Duronio, R.J.; Brook, A.; Dyson, N.; O’Farrell, P.H. E2F-Induced S Phase Requires Cyclin E. Genes Dev. 1996, 10, 2505–2513. [CrossRef]
- Oakes, V.; Wang, W.; Harrington, B.; Lee, W.J.; Beamish, H.; Chia, K.M.; Pinder, A.; Goto, H.; Inagaki, M.; Pavey, S.; et al. Cyclin A/Cdk2 Regulates Cdh1 and Claspin during Late S/G2 Phase of the Cell Cycle. Cell Cycle 2014, 13, 3302–3311. [CrossRef]
- Vigneron, S.; Sundermann, L.; Labbé, J.C.; Pintard, L.; Radulescu, O.; Castro, A.; Lorca, T. Cyclin A-Cdk1-Dependent Phosphorylation of Bora Is the Triggering Factor Promoting Mitotic Entry. Dev. Cell 2018, 45, 637-650.e7. [CrossRef]
- Gavet, O.; Pines, J. Activation of Cyclin B1-Cdk1 Synchronizes Events in the Nucleus and the Cytoplasm at Mitosis. J. Cell Biol. 2010, 189, 247–259. [CrossRef]
- Li, B.; Zhou, P.; Xu, K.; Chen, T.; Jiao, J.; Wei, H.; Yang, X.; Xu, W.; Wan, W.; Xiao, J. Metformin Induces Cell Cycle Arrest, Apoptosis and Autophagy through ROS/JNK Signaling Pathway in Human Osteosarcoma. Int. J. Biol. Sci. 2020, 16, 74–84. [CrossRef]
- McConnell, B.B.; Gregory, F.J.; Stott, F.J.; Hara, E.; Peters, G. Induced Expression of P16 INK4a Inhibits Both CDK4- and CDK2-Associated Kinase Activity by Reassortment of Cyclin-CDK-Inhibitor Complexes . Mol. Cell. Biol. 1999, 19, 1981–1989. [CrossRef]
- Qin, X.Q.; Livingston, D.M.; Kaelin, W.G.; Adams, P.D. Deregulated Transcription Factor E2F-1 Expression Leads to S-Phase Entry and P53-Mediated Apoptosis. Proc. Natl. Acad. Sci. U. S. A. 1994, 91, 10918–10922. [CrossRef]
- Huang, Y.C.; Lee, P.C.; Wang, J.J.; Hsu, Y.C. Anticancer Effect and Mechanism of Hydroxygenkwanin in Oral Squamous Cell Carcinoma. Front. Oncol. 2019, 9, 1–10. [CrossRef]
- Fonseca, A.C.C. da; de Queiroz, L.N.; Sales Felisberto, J.; Jessé Ramos, Y.; Mesquita Marques, A.; Wermelinger, G.F.; Pontes, B.; de Lima Moreira, D.; Robbs, B.K. Cytotoxic Effect of Pure Compounds from Piper Rivinoides Kunth against Oral Squamous Cell Carcinoma. Nat. Prod. Res. 2021, 35, 6163–6167. [CrossRef]
- de Campos, P.S.; Matte, B.F.; Diel, L.F.; Jesus, L.H.; Bernardi, L.; Alves, A.M.; Rados, P.V.; Lamers, M.L. Low Doses of Curcuma Longa Modulates Cell Migration and Cell–Cell Adhesion. Phyther. Res. 2017, 31, 1433–1440. [CrossRef]
- Chourasia, N.R.; Borle, R.M.; Vastani, A. Concomitant Association of Oral Submucous Fibrosis and Oral Squamous Cell Carcinoma and Incidence of Malignant Transformation of Oral Submucous Fibrosis in a Population of Central India: A Retrospective Study. J. Maxillofac. Oral Surg. 2015, 14, 902–906. [CrossRef]
- Evren, I.; Brouns, E.R.; Wils, L.J.; Poell, J.B.; Peeters, C.F.W.; Brakenhoff, R.H.; Bloemena, E.; de Visscher, J.G.A.M. Annual Malignant Transformation Rate of Oral Leukoplakia Remains Consistent: A Long-Term Follow-up Study. Oral Oncol. 2020, 110, 105014. [CrossRef]
- Iocca, O.; Sollecito, T.P.; Alawi, F.; Weinstein, G.S.; Newman, J.G.; De Virgilio, A.; Di Maio, P.; Spriano, G.; Pardiñas López, S.; Shanti, R.M. Potentially Malignant Disorders of the Oral Cavity and Oral Dysplasia: A Systematic Review and Meta-Analysis of Malignant Transformation Rate by Subtype. Head Neck 2020, 42, 539–555. [CrossRef]
- Lodi, G.; Franchini, R.; Warnakulasuriya, S.; Varoni, E.M.; Sardella, A.; Kerr, A.R.; Carrassi, A.; MacDonald, L.C.; Worthington, H. V.; Mauleffinch, L.F. Interventions for Treating Oral Leukoplakia to Prevent Oral Cancer (Review). Cochrane Database Syst. Rev. 2016. [CrossRef]
- Papadimitrakopoulou, V.A.; William, W.N.; Dannenberg, A.J.; Lippman, S.M.; Lee, J.J.; Ondrey, F.G.; Peterson, D.E.; Feng, L.; Atwell, A.; El-Naggar, A.K.; et al. Pilot Randomized Phase II Study of Celecoxib in Oral Premalignant Lesions. Clin. Cancer Res. 2008, 14, 2095–2101. [CrossRef]
- William, W.N.; Papadimitrakopoulou, V.; Lee, J.J.; Mao, L.; Cohen, E.E.W.; Lin, H.Y.; Gillenwater, A.M.; Martin, J.W.; Lingen, M.W.; Boyle, J.O.; et al. Erlotinib and the Risk of Oral Cancer the Erlotinib Prevention of Oral Cancer (EPOC) Randomized Clinical Trial. JAMA Oncol. 2016, 2, 209–216. [CrossRef]
- Gutkind, J.S.; Molinolo, A.A.; Wu, X.; Wang, Z.; Nachmanson, D.; Harismendy, O.; Alexandrov, L.B.; Wuertz, B.R.; Ondrey, F.G.; Laronde, D.; et al. Inhibition of MTOR Signaling and Clinical Activity of Metformin in Oral Premalignant Lesions. JCI Insight 2021, 6. [CrossRef]
- Tian, H.; Lyu, Y.; Yang, Y.G.; Hu, Z. Humanized Rodent Models for Cancer Research. Front. Oncol. 2020, 10, 1–11. [CrossRef]
- Bouaoud, J.; De Souza, G.; Darido, C.; Tortereau, A.; Elkabets, M.; Bertolus, C.; Saintigny, P. The 4-NQO Mouse Model: An Update on a Well-Established in Vivo Model of Oral Carcinogenesis. Methods Cell Biol. 2021, 163, 197–229. [CrossRef]
- Schoop, R.A.L.; Noteborn, M.H.M.; Baatenburg De Jong, R.J. A Mouse Model for Oral Squamous Cell Carcinoma. J. Mol. Histol. 2009, 40, 177–181. [CrossRef]
- Wang, J.; Wang, S.; Wang, Y.; Wang, L.; Xia, Q.; Tian, Z.; Guan, X. Chemopreventive Effect of Modified Zengshengping on Oral Cancer in a Hamster Model and Assessment of Its Effect on Liver. J. Ethnopharmacol. 2020, 255, 1–9. [CrossRef]
- Dai, Z.; Zhu, P.F.; Liu, H.; Li, X.C.; Zhu, Y.Y.; Liu, Y.Y.; Shi, X.L.; Chen, W. Di; Liu, Y.P.; Zhao, Y. li; et al. Discovery of Potent Immune-Modulating Molecule Taccaoside A against Cancers from Structures-Active Relationships of Natural Steroidal Saponins. Phytomedicine 2022, 104, 154335. [CrossRef]
- Cattaneo, C.M.; Dijkstra, K.K.; Fanchi, L.F.; Kelderman, S.; Kaing, S.; van Rooij, N.; van den Brink, S.; Schumacher, T.N.; Voest, E.E. Tumor Organoid–T-Cell Coculture Systems. Nat. Protoc. 2020, 15, 15–39. [CrossRef]
- Neal, J.T.; Li, X.; Zhu, J.; Giangarra, V.; Grzeskowiak, C.L.; Ju, J.; Liu, I.H.; Chiou, S.H.; Salahudeen, A.A.; Smith, A.R.; et al. Organoid Modeling of the Tumor Immune Microenvironment. Cell 2018, 175, 1972-1988.e16. [CrossRef]
- Zhou, Z.; Van der Jeught, K.; Fang, Y.; Yu, T.; Li, Y.; Ao, Z.; Liu, S.; Zhang, L.; Yang, Y.; Eyvani, H.; et al. An Organoid-Based Screen for Epigenetic Inhibitors That Stimulate Antigen Presentation and Potentiate T-Cell-Mediated Cytotoxicity. Nat. Biomed. Eng. 2021, 5, 1320–1335. [CrossRef]
- Verma, A.; Vincent-chong, V.K.; Dejong, H.; Hershberger, P.A.; Seshadri, M.; Park, R.; Cancer, C.; Park, R.; Cancer, C.; Prosthetics, M.; et al. Impact of Dietary Vitamin D on Initiation and Progression of Oral Cancer. J Steroid Biochem Mol Biol 2020, 199, 1–23. [CrossRef]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2020, 10, 1–17. [CrossRef]
Figure 1.
Natural products involved in preclinical trials involving HNSCC cell lines and animal models
Figure 1.
Natural products involved in preclinical trials involving HNSCC cell lines and animal models
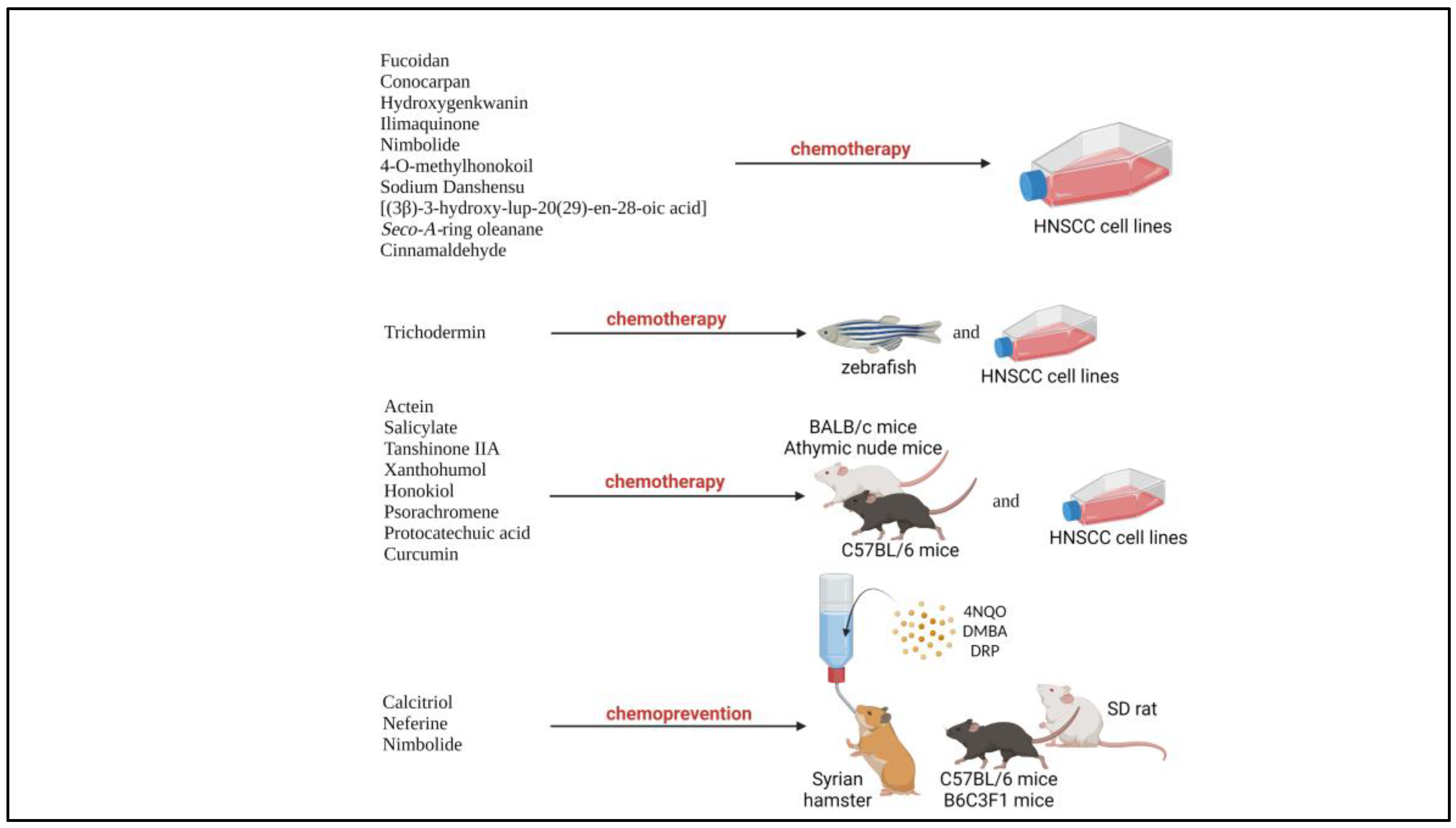
Figure 2.
Various natural products and their respective molecular mechanisms against HNSCC; (natural product name in green) chemoprevention, (natural product name in black) chemotherapeutic
Figure 2.
Various natural products and their respective molecular mechanisms against HNSCC; (natural product name in green) chemoprevention, (natural product name in black) chemotherapeutic
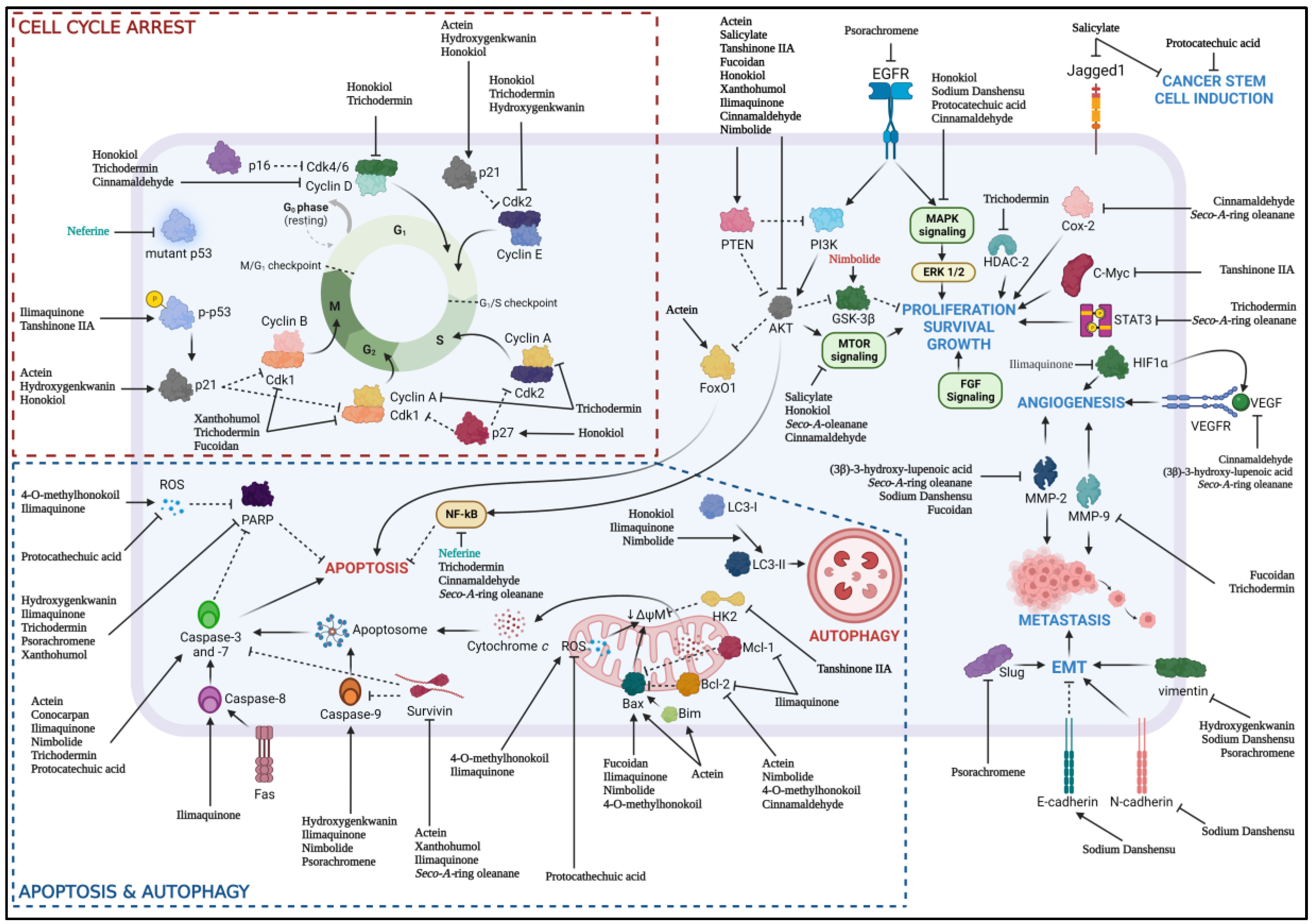
Table 1.
Chemotherapeutic findings from preclinical studies with natural products involving in vitro HNSCC cell lines.
Table 1.
Chemotherapeutic findings from preclinical studies with natural products involving in vitro HNSCC cell lines.
| No | Author | PMID | Natural product | Preclinical Model (cell lines) |
Findings | Pathway related |
|---|---|---|---|---|---|---|
| 1 | Zhang et al. [49] |
31495936 | Fucoidan | In vitro (SCC15, SCC25) |
|
Akt Bax/Bcl-2 |
| 2 | Fonseca et al. [107] |
33078660 | Conocarpan | In vitro (SCC9, SCC4, SCC25) |
|
Caspase |
| 3 | Huang et al. [106] |
31620368 | Hydroxygenkwanin | In vitro (SAS & OECM1) |
|
Caspase |
| 4 | Lin et al. [46] |
32825464 | Ilimaquinone | In vitro (SCC4, SCC2095) |
|
Akt Caspase Bax/Bcl-2 |
| 5 | Sophia et al. [47] |
30352996 | Nimbolide | In vitro (SCC131, SCC4) |
|
PI3K/Akt/GSK-3β Caspase Bax/Bcl-2 |
| 6 | Xiao et al. [91] |
29332355 | 4-O-methylhonokoil | In vitro (PE/CA-PJ41) |
|
Bax/Bcl-2 |
| 7 | Kumar et al. [55] |
33101201 | Sodium Danshensu | In vitro (FaDu, CA9-22) |
|
MAPK/ERK |
| 8 | Aswathy et al. [50] |
33860206 | [(3β)-3-hydroxy-lup-20(29)-en-28-oic acid] | In vitro (SAS) |
|
Akt/mTOR JAK/STAT3 VEGF NF-κB |
| Seco-A-ring oleanane | In vitro (SAS) |
|
||||
| 9 | Aggarwal et al. [48] |
35774603 | Cinnamaldehyde | In vitro (SCC9, SCC25, SCC4) |
|
PI3K/Akt/mTOR NF-κB MAPK |
Table 2.
Chemotherapeutic findings from preclinical studies with natural products involving in vitro HNSCC cell lines and in vivo xenograft models
Table 2.
Chemotherapeutic findings from preclinical studies with natural products involving in vitro HNSCC cell lines and in vivo xenograft models
| No | Author | PMID | Natural product | Preclinical model |
Findings | Pathway related |
|---|---|---|---|---|---|---|
| 1 | Zhao et al. [41] |
34175854 | Actein | In vitro (CAL27, SCC9) |
|
Akt/FoxO1 Bax/Bcl-2 |
|
In vivo (CAL27) C57BL/6 mice |
|
|||||
| 2 | Zhang et al. [42] |
32267053 | Salicylate | In vitro (SAS) |
|
Akt/mTOR |
|
In vivo (SAS) Nude mice |
|
|||||
| 3 | Li et al. [43] |
32424132 | Tanshinone IIA | In vitro (CAL27, SCC9, SCC15, SCC25) |
|
Akt/c-Myc |
|
In vivo (CAL27, SCC15) Athymic nude mice |
|
|||||
| 4 | Li et al. [44] |
32410646 | Xanthohumol | In vitro (CAL27, SCC9, SCC15, SCC25) |
|
Akt/Wee1/Cdk1 Bax/Bcl-2 Caspase |
|
In vivo (CAL27, SCC25) Athymic nude mice |
|
|||||
| 5 | Huang et al. [45] |
29363886 | Honokiol | In vitro (OC2, OCSL) |
|
Akt/mTOR MAPK |
|
In vivo (SAS) BALB/c nude mice, AnN.Cg-Foxn1nu/CrlNarl |
|
|||||
| 6 | Chen et al. [65] |
35785707 | Trichodermin | In vitro (Ca922, HSC3) |
|
HDAC-2 Caspase |
|
In vivo (HSC3) Zebrafish |
|
|||||
| 7 | Wang et al. [24] |
31750253 | Psorachromene | In vitro (SAS, OECM1) |
|
EGFR EMT-related Caspase SLUG |
|
In vivo (SAS) BALB/c nude mice |
|
|||||
| 8 | Li et al. [56] |
35904511 | Protocatechuic acid | In vitro (HSC3, CAL27) |
|
JNK/p38 Caspase |
|
In vivo (CAL27) Nude mice |
|
|||||
| 9 | de Compos et al. [108] |
28782139 | Curcumin |
In vitro (CAL27, SCC25, HACAT, NIH-3T3) |
|
|
|
In vivo (HNSCC Biopsy) BALB/c nude mice |
|
Table 3.
Chemoprevention findings from preclinical studies with natural products on induced carcinogenesis.
Table 3.
Chemoprevention findings from preclinical studies with natural products on induced carcinogenesis.
| No | Author | PMID | Natural product | Preclinical model |
Findings | Pathway related |
|---|---|---|---|---|---|---|
| 1 | Vincent-Chong et al. [25] |
30875566 | Calcitriol |
In vivo (4NQO-induced carcinogenesis) C57BL/6NCr mice |
|
|
| 2 | Wang et al. [64] |
33156559 | Neferine |
In vivo (DMBA-induced carcinogenesis) Syrian hamster |
|
NF-κB |
| 3 | Sophia et al. [47] |
30352996 | Nimbolide |
In vivo (DMBA-induced carcinogenesis) Syrian hamster |
|
PI3K/Akt/GSK-3β |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Molecular Aspects of Piperine in Signaling Pathways Associated with Inflammation in Head and Neck Cancer
Juliana Prado Gusson-Zanetoni
et al.
,
2024
The Use of Chinese Herbal Products for Nasopharyngeal Carcinoma in Taiwan: A Population-Based Study
Shih-Ting Tseng
et al.
,
2018
Cytotoxic Potential of the Monoterpene Isoespintanol against Human Tumor Cell Lines
Orfa Inés Contreras-Martínez
et al.
,
2024
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated
