Modulation of the RAS Beyond ACE Inhibition
ACE inhibitors have emerged as the treatment of choice for patients with all degrees of heart failure. The first ACE inhibitor trial to show a reduction in mortality in patients with heart failure was the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS)[10] which demonstrated that treatment with enal-april resulted in significant decreased mortality. The Survival and Ventricular Enlargement (SAVE) trial[21] was first to show that an ACE inhibitor could reduce mortality and limit remodeling after MI. In this trial[21] patients who were relatively asymptomatic after a first infarction received either captopril or placebo. The reduction in mortality was accompanied by a reduction in left ventricular dilatation, recurrent MI, and cardiovascular mortality. Several subsequent studies have confirmed this post-MI benefit of RAS inhibition.
The Heart Outcomes Prevention Evaluation study (HOPE)[9] recently showed that ACE inhibition with ramipril reduces the risk of a primary cardiovascular event in high-risk patients by 22% ( Table ). This benefit was greater than expected from the observed decrease in blood pressure and suggested that ramipril has protective effects on the vasculature and other target organs beyond the blood pressure-lowering effect.
Because of the pivotal role of angiotensin II in the different steps of the cardiovascular continuum, more complete inhibition of the RAS to reduce the overall cardiovascular risk of an individual is a logical hypothesis that needs to be tested.
In theory, the effects on vasculature and clinical outcome might be even greater with ARBs as compared to ACE inhibitors since ARBs might block angiotensin II formed by non-ACE pathways and the negative effects of angiotensin II are selectively blocked while the favorable effects of the AT2R are preserved with ARBs.
Various clinical trials have investigated long-term treatment effects of ARBs on mortality and morbidity in humans. The Reduction of Endpoints in Non-Insulin Dependent Diabetes Mellitus With the Angiotensin II Antagonist Losartan (RENAAL) trial[11] indicated that losartan provided significant renal protection independent of blood pressure-lowering effects. Although the smaller Evaluation of Losartan in the Elderly (ELITE-I) study[29] indicated the possibility of improved survival with the ARB than with the ACE inhibitor, the larger ELITE-II study[30] was unable to demonstrate any benefit of losartan treatment compared to captopril among 3152 patients with New York Heart Association (NYHA) class II-IV heart failure. However, the dose and dosing frequency of losartan (50 mg daily) might have been too low to effectively inhibit the AT1R expression. Furthermore, ELITE-II was neither designed nor powered to test for equivalence or noninferiority; therefore, the results of ELITE-II suggest that ARBs should not replace ACE inhibitors as an alternative manner of suppressing the RAS in CHF.
The role of adding an ARB to existing treatment with ACE inhibitors has not been proved, but is an important question since several studies have demonstrated that a significant amount of angiotensin II production results from non-ACE production. Nishimoto and colleagues[31] were able to show that chymase-dependent angiotensin II formation plays an important role in the development of vascular proliferation in grafted veins. The clinical significance of these results is important. Since ACE inhibitors may not be capable of suppressing chymase-dependent angiotensin II formation, treatment with ARBs might fill in this gap and guarantee complete inhibition of the RAS.
Experimental data in sheep treated with ACE inhibitors, ARBs alone or in combination after MI revealed that the dual therapy was slightly superior to a single treatment with ACE inhibitors or ARBs alone regarding postinfarction left ventricular remodeling (Figure 2).[32]
Experimental data in sheep treated with angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARBs) alone, or in combination after myocardial infarction revealed that the dual therapy was slightly superior to a single treatment with ACEI or ARB alone regarding postinfarction left ventricular remodeling.Reprinted with permission from Circulation. 2001;103:2845-2850.[32]LV ESVI=left ventricular end-systolic volume indices; AT1=angiotensin II type 1 antagonism; CT=combination therapy; *p<0.04 vs. control; p<0.05 vs. control
Several dual blockade trials (ACE inhibitor and/or ARB) in heart failure in humans have shown more promising results. The relatively small, short-term, placebo controlled Vasodilator Heart Failure Trial (V-HeFT)[33] found augmented effects on blood pressure as well as hormonal and hemodynamic factors after adding an ARB to a preexisting ACE inhibitor medication in patients with CHF. In the Randomized Evaluation of Strategies for Left Ventricular Dysfunction (RESOLVD) pilot study[34] 768 patients with NYHA II-IV and an ejection fraction of less than 40% were randomized to either candesartan or enalapril or combined candesartan and enalapril. The combination therapy was more effective in preventing left ventricular remodeling than candesartan or enalapril alone. Surprisingly, the Valsartan Heart Failure Trial (Val-HeFT),[35] which used valsartan (target dose 160 mg twice daily) added to an existing heart failure medication including ACE inhibitors and
 blockers, showed that patients with a combination of ACE inhibitors and ARBs did not significantly benefit from a dual treatment (Figure 3). There was, however, a relative risk reduction in the combined end point mortality/morbidity of approximately 13% (p=0.009) in the valsartan group, mainly due to a 27% reduction in the risk of CHF hospitalization. In addition, significant improvements in other secondary end points like quality of life and left ventricular ejection fraction in the valsartan group were achieved. Unexpectedly, the ad hoc subgroup analysis[35] revealed that patients treated with valsartan, ACE inhibitors, and
blockers, showed that patients with a combination of ACE inhibitors and ARBs did not significantly benefit from a dual treatment (Figure 3). There was, however, a relative risk reduction in the combined end point mortality/morbidity of approximately 13% (p=0.009) in the valsartan group, mainly due to a 27% reduction in the risk of CHF hospitalization. In addition, significant improvements in other secondary end points like quality of life and left ventricular ejection fraction in the valsartan group were achieved. Unexpectedly, the ad hoc subgroup analysis[35] revealed that patients treated with valsartan, ACE inhibitors, and
 blockers even had a 10% increase in the combined end point of all-cause mortality and morbidity compared to ACE inhibitors and
blockers even had a 10% increase in the combined end point of all-cause mortality and morbidity compared to ACE inhibitors and
 blockers alone. This trend toward an increased rate of mortality and morbidity events in the
blockers alone. This trend toward an increased rate of mortality and morbidity events in the
 blocker subgroup raised great concerns since
blocker subgroup raised great concerns since
 blockers, along with ACE inhibitors, are considered first-line therapy for patients with CHF. However, there is no biologically plausible explanation for adverse interactions between
blockers, along with ACE inhibitors, are considered first-line therapy for patients with CHF. However, there is no biologically plausible explanation for adverse interactions between
 blockers and ARBs and/or ACE inhibitors; therefore, it must be emphasized that this was a retrospective subgroup analysis which requires further studies.[35] The results of the Valsartan in Acute Myocardial Infarction Trial (VALIANT),[36] testing valsartan alone or in combination with an ACE inhibitor in patients with acute MI, will provide further insight since more than 70% of the patients have had conventional post-MI therapy with
blockers and ARBs and/or ACE inhibitors; therefore, it must be emphasized that this was a retrospective subgroup analysis which requires further studies.[35] The results of the Valsartan in Acute Myocardial Infarction Trial (VALIANT),[36] testing valsartan alone or in combination with an ACE inhibitor in patients with acute MI, will provide further insight since more than 70% of the patients have had conventional post-MI therapy with
 blockers.
blockers.
The Valsartan Heart Failure Trial (Val-HeFT), which used valsartan added to an existing heart failure medication including angiotensin-converting enzyme (ACE) inhibitors and blockers, showed that patients with a combination of ACE inhibitors and angiotensin receptor blockers had a 13.3% risk reduction in the combined primary end point mortality and morbidity.
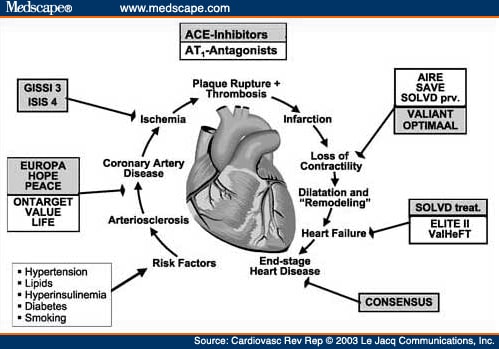
The issue of using ACE inhibitors in combination with ARBs will probably not be resolved until the results of major ongoing trials are published. The Candesartan in Heart Failure-Assessment of Reduction in Mortality and Morbidity (CHARM)[37] will evaluate the effects of candesartan on all-cause mortality in symptomatic heart failure with or without ACE inhibitor treatment. The treatment of 14,500 patients with prior MI and heart failure with valsartan, captopril, or the combination of both will be assessed by the VALIANT trial.[36] Finally, the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial (ONTARGET)[38] will compare the efficacy of telmisartan with ramipril, either alone or in combination, in preventing cardiovascular end points (Figure 4).
Pharmacologic interventions to cover the gaps in vascular protection. Completed and ongoing studies evaluating the benefit of angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers alone or in combination and their influence on the different steps of the cardiovascular continuumGISSI 3=Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico 3; ISIS 4=International Study of Infarct Survival 4; EUROPA=European Trial on Reduction of Cardiac Events With Perindopril in Stable Coronary Artery Disease; PEACE=Prevention of Events With Angiotensin-Converting Enzyme Inhibition; VALUE=The Valsartan Antihypertensive Long-term Use Evaluation; AIRE=Acute Infarction Ramipril Efficacy; SOLVD prv=Study of Left Ventricular Dysfunction-prevention; OPTIMAAL=Optimal Trial in Myocardial Infarction With Angiotensin II Antagonist Losartan. Other trial acronyms expanded in text.
Cardiovasc Rev Rep. 2003;24(4) © 2003 Le Jacq Communications, Inc.
Cite this: Inhibition of the Renin-Angiotensin System and Vascular Protection - Medscape - Apr 01, 2003.