Ibogaine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Lambarène, Iperton[1][2] |
| Other names | 12-Methoxyibogamine |
| Routes of administration | Oral |
| Drug class | Hallucinogen; Oneirogen; Entheogen; Stimulant; Antiaddictive agent |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.001.363 |
| Chemical and physical data | |
| Formula | C20H26N2O |
| Molar mass | 310.441 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 152 to 153 °C (306 to 307 °F) |
| |
| |
| (verify) | |
Ibogaine is a psychoactive indole alkaloid obtained either by extraction from plants in the family Apocynaceae such as Tabernanthe iboga, Voacanga africana, and Tabernaemontana undulata or by semi-synthesis from the precursor compound voacangine, another plant alkaloid.[1][2] The total synthesis of ibogaine was described in 1956.[5] Structural elucidation by X-ray crystallography was completed in 1960.[6][7][8]
The psychoactivity of the root bark of the iboga tree, Tabernanthe iboga, one of the plants from which ibogaine is extracted, was first discovered by the Pygmy tribes of Central Africa, who passed the knowledge to the Bwiti tribe of Gabon. French explorers in turn learned of it from the Bwiti tribe and brought ibogaine back to Europe in 1899–1900, where it was subsequently marketed in France as a stimulant under the trade name Lambarène until the 1960s.[1][2] It was also marketed as Iperton.[2] Although ibogaine's anti-addictive properties were first widely promoted in 1962 by Howard Lotsof, its Western medical use predates that by at least a century.
During an eighteen-year timeline, a total of 19 fatalities temporally associated with the ingestion of ibogaine were reported, from which six subjects died of acute heart failure or cardiopulmonary arrest. Its prohibition in many countries has slowed scientific research.[9] Various derivatives of ibogaine designed to lack psychedelic properties, such as 18-MC, are under preliminary research.
Psychoactive effects
[edit]Ibogaine is derived from the root of Tabernanthe iboga, a plant known to exhibit psychedelic effects in its users.[10] The experience of ibogaine occurs in two phases, termed the visionary phase and the introspection phase. The visionary phase has been described as oneirogenic, referring to the dreamlike nature of its psychedelic effects, and lasts for 4 to 6 hours. The second phase, the introspection phase, is responsible for the psychotherapeutic effects.[citation needed] It can allow people to conquer their fears and negative emotions.[citation needed] Ibogaine catalyzes an altered state of consciousness reminiscent of dreaming while fully conscious and aware so that memories, life experiences, and issues of trauma can be processed.[11]
Uses
[edit]
Medical
[edit]Clinical studies of ibogaine to treat drug addiction began in the early 1990s, but concerns about cardiotoxicity terminated those studies.[12] A 2022 review indicated that severe adverse effects, including deaths, have impeded progress toward clinical adoption of ibogaine for use in opioid abstinence.[13] There is insufficient evidence to determine whether ibogaine is useful for treating addiction.[13][14]
Adverse effects
[edit]Immediate adverse effects of ibogaine ingestion may include nausea, tremors leading to ataxia, headaches, and mental confusion.[15] In long-term use, manic episodes may last for several days, possibly including insomnia, irritability, emotional instability, delusions, aggressive behavior, and thoughts of suicide.[15] In the heart, ibogaine causes long QT syndrome at higher doses, apparently by blocking hERG potassium channels and slowing the heart rate.[16][17] Ibogaine should not be used during pregnancy or breastfeeding.[15]
Ibogaine has potential for adverse interactions with other psychedelic agents and prescription drugs.[15][17]
Death may occur with the use of ibogaine,[17] especially if consumed with opioids or in people with existing morbidities, such as cardiovascular disease or neurological disorders.[15]
Neurotoxicity
[edit]Laboratory studies in rats indicate that high-dose ibogaine may cause degeneration of cerebellar Purkinje cells.[18] However, subsequent research found no evidence of neurotoxicity in a primate.[19]
In limited human research, neuropathological examination revealed no evidence of neuronal degenerative changes in a woman who had received four separate doses of ibogaine ranging between 10 and 30 mg⁄ kg over a 15-month interval.[19] A published series of fatalities associated with ibogaine ingestion found no evidence for consistent neurotoxicity.[20]
Pharmacology
[edit]Pharmacodynamics
[edit]| Site | Ibogaine | Noribogaine |
|---|---|---|
| MOR | 2,000–100,000 | 700–3,000 |
| DOR | >100,000 | 5,000–25,000 |
| KOR | 2,000–4,000 | 600–1,000 |
| 5-HT2A | 16,000 | >100,000 |
| 5-HT2C | >10,000 | >10,000 |
| 5-HT3 | 2,600 | >100,000 |
| σ1 | 2,500–9,000 | 11,000–15,000 |
| σ2 | 90–400 | 5,000–19,000 |
| NMDA | 1,000–3,000 | 6,000–15,000 |
| nACh | 20 | 1,500 |
| SERT | 500 | 40 |
| DAT | 2,000 | 2,000 |
| Values are Ki (nM). The smaller the value, the more strongly the drug binds to the site. | ||
Ibogaine affects many different neurotransmitter systems simultaneously.[23][24]
Noribogaine is most potent as a serotonin reuptake inhibitor. It acts as a moderate κ-opioid receptor agonist[25] and weak μ-opioid receptor agonist[25] or weak partial agonist.[26] It is possible that the action of ibogaine at the kappa opioid receptor may indeed contribute significantly to the psychoactive effects attributed to ibogaine ingestion; Salvia divinorum, another plant recognized for its strong hallucinogenic properties, contains the chemical salvinorin A, which is a highly selective kappa opioid agonist. Noribogaine is more potent than ibogaine in rat drug discrimination assays when tested for the subjective effects of ibogaine.[27]
Pharmacokinetics
[edit]Ibogaine is metabolized in the human body by cytochrome P450 2D6 (CYP2D6) into noribogaine (more correctly, O-desmethylibogaine or 12-hydroxyibogamine). Both ibogaine and noribogaine have a plasma half-life of around two hours in rats,[28] although the half-life of noribogaine is slightly longer than that of the parent compound. It is proposed that ibogaine is deposited in fat and metabolized into noribogaine as it is released.[29] After ibogaine ingestion in humans, noribogaine shows higher plasma levels than ibogaine and is detected for a longer period of time than ibogaine.[30]
Chemistry
[edit]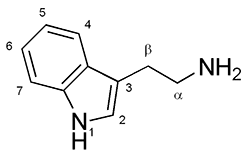
Ibogaine is a substituted tryptamine. It has two separate chiral centers, meaning that there are four different stereoisomers of ibogaine. These four isomers are difficult to resolve.[31]
Synthesis
[edit]One recent total synthesis[32] of ibogaine and related drugs starts with 2-iodo-4-methoxyaniline which is reacted with triethyl((4-(triethylsilyl)but-3-yn-1-yl)oxy)silane using palladium acetate in DMF to form 2-(triethylsilyl)-3-(2-((triethylsilyl)oxy)ethyl)-1H-indole. This is converted using N-iodosuccinamide and then fluoride to form 2-(2-iodo-1H-indol-3-yl)ethanol. This is treated with iodine, triphenyl phosphine, and imidazole to form 2-iodo-3-(2-iodoethyl)-1H-indole. Then, using 7-ethyl-2-azabicyclo[2.2.2]oct-5-ene and cesium carbonate in acetonitrile, the ibogaine precursor 7-ethyl-2-(2-(2-iodo-1H-indol-3-yl)ethyl)-2-azabicyclo[2.2.2]oct-5-ene is obtained. Using palladium acetate in DMF, the ibogaine is obtained. If the exo ethyl group on the 2-azabicyclo[2.2.2]octane system in ibogaine is replaced with an endo ethyl, then epiibogaine is formed.
Crystalline ibogaine hydrochloride is typically produced by semi-synthesis from voacangine in commercial laboratories.[33][34] It can be prepared from voacangine through one-step demethoxycarbonylation process too.[35]
Derivatives
[edit]A synthetic derivative of ibogaine, 18-methoxycoronaridine (18-MC), is a selective α3β4 antagonist that was developed collaboratively by the neurologist Stanley D. Glick (Albany) and the chemist Martin E. Kuehne (Vermont).[36] This discovery was stimulated by earlier studies on other naturally occurring analogues of ibogaine, such as coronaridine and voacangine, that showed these compounds to have anti-addictive properties.[37][38] More recently, non- and less-hallucinogenic analogs, tabernanthalog and ibogainalog, were engineered by scientists attempting to produce non-cardiotoxic ibogaine derivatives by removing the lipophilic isoquinuclidine ring. In animal models, both molecules failed to produce cardiac arrhythmias, and tabernanthalog failed to produce any head twitch response, suggesting psychedelic effects were absent.[39][40]
Biosynthesis
[edit]
Ibogaine biosynthesis begins with tryptophan undergoing enzymatic decarboxylation by tryptophan decarboxylase (TDC) to form a tryptamine. Secologanin, an iridoid synthesized from isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP), is reacted with tryptamine to make strictosidine. A glycosidic bond cleavage of strictosidine by strictosidine β-deglucosidase (SGD) produces a lactol. The lactol opens and produces an aldehyde, then condenses to form an iminium. Through isomerization and reduction by geissoschizine synthase 1 (GS1), 19E-geissoschizine is yielded. The indole is oxidized and the molecule undergoes intramolecular Mannich reaction and Grob fragmentation to form preakuammicine. Preakuammicine is highly unstable and therefore reduced to stemmadenine by oxidation-reduction reactions (REDOX 1 and REDOX 2). Stemmadine is acylated by stemmadine Ο-acetyltransferase (SAT) to yield stemmadine acetate. Through oxidation by precondylocarpine acetate synthase (PAS) and reduction by dihydroprecondylocarpine acetate synthase (DPAS), an enamine intermediate is formed. The intermediate undergoes fragmentation to produce an iminium that tautomerizes to yield dehydrosecodine. Coronaridine synthase (CorS) catalyzes the isomerization of dehydrosecodine and an unusual cycloaddition is completed. The iminium is reduced by DPAS and NADPH to form (-)-coronaridine.[citation needed]
There are two pathways (-)-coronaridine can take to become (-)-ibogaine. The first pathway begins with a P450 enzyme, ibogamine-10-hydroxylase (I10H), and methylation of noribogaine-10-Ο-methyltransferase (N10OMT) to produce (-)-voacangine. Polyneudridine aldehyde esterase-like 1 (PNAE1) and a spontaneous decarboxylation can convert (-)-voacangine to (-)-ibogaine. The second pathway consists of PNAE1 and the spontaneous decarboxylation occurring first to yield (-)-ibogamine, then the reaction of I10H-mediated hydroxylation and N10OMT-catalyzed O-methylation to produce (-)-ibogaine.[41]
Natural occurrence
[edit]Ibogaine occurs naturally in iboga root bark. Ibogaine is also available in a total alkaloid extract of the Tabernanthe iboga plant, which also contains all the other iboga alkaloids and thus has only about half the potency by weight of standardized ibogaine hydrochloride.[33]
History
[edit]The use of iboga in African spiritual ceremonies was first reported by French and Belgian explorers in the 19th century, beginning with the work of French naval physician and explorer of Gabon Marie-Théophile Griffon du Bellay.[42] The first botanical description of the Tabernanthe iboga plant was made in 1889. Ibogaine was first isolated from T. iboga in 1901 by Dybowski and Landrin[43] and independently by Haller and Heckel in the same year using T. iboga samples from Gabon. Complete synthesis of ibogaine was accomplished by G. Büchi in 1966.[44] Since then, several other synthesis methods have been developed.[45]
From the 1930s to 1960s, ibogaine was sold in France in the form of Lambarène, an extract of the Tabernanthe manii plant, and promoted as a mental and physical stimulant.[1] It was formulated at doses of 200 mg extract containing low doses of 4 to 8 mg ibogaine per tablet.[2][1] The drug enjoyed some popularity among post-World War II athletes. Lambarène was withdrawn from the market in 1966 when the sale of ibogaine-containing products became illegal in France.[46][1] Another formulation was Iperton, which contained Tabernanthe iboga extract 40 mg per dose unit.[2]
In 2008, Mačiulaitis and colleagues stated that in the late 1960s, the World Health Assembly classified ibogaine as a "substance likely to cause dependency or endanger human health". The U.S. Food and Drug Administration (FDA) also assigned it to a Schedule I classification, and the International Olympic Committee banned it as a potential doping agent.[2]
Anecdotal reports concerning ibogaine's effects appeared in the early 1960s.[47] Its anti-addictive properties were discovered accidentally by Howard Lotsof in 1962, at the age of 19, when he and five friends—all heroin addicts—noted subjective reduction of their craving and withdrawal symptoms while taking it.[48] Further anecdotal observation convinced Lotsof of its potential usefulness in treating substance addictions. He contracted with a Belgian company to produce ibogaine in tablet form for clinical trials in the Netherlands, and was awarded a United States patent for the product in 1985. The first objective, placebo-controlled evidence of ibogaine's ability to attenuate opioid withdrawal in rats was published by Dzoljic et al. in 1988.[49] Diminution of morphine self-administration was reported in preclinical studies by Glick et al. in 1991.[50] Cappendijk et al. demonstrated reduction in cocaine self-administration in rats in 1993,[51] and Rezvani reported reduced alcohol dependence in three strains of "alcohol-preferring" rats in 1995.[52]
As the use of ibogaine spread, its administration varied widely; some groups administered it systematically using well-developed methods and medical personnel, while others employed haphazard and possibly dangerous methodology. Lotsof and his colleagues, committed to the traditional administration of ibogaine, developed treatment regimens themselves. In 1992, Eric Taub brought ibogaine to an offshore location close to the United States, where he began providing treatments and popularizing its use.[53] In Costa Rica, Lex Kogan, another leading proponent, joined Taub in systematizing its administration. The two men established medically monitored treatment clinics in several countries.[54]
In 1981, an unnamed European manufacturer produced 44 kg of iboga extract. The entire stock was purchased by Carl Waltenburg, who distributed it under the name "Indra extract" and used it in 1982 to treat heroin addicts in the community of Christiania.[10] Indra extract was available for sale over the Internet until 2006, when the Indra web presence disappeared. Various products are currently sold in a number of countries as "Indra extract", but it is unclear if any of them are derived from Waltenburg's original stock. Ibogaine and related indole compounds are susceptible to oxidation over time.[55][56]
The National Institute on Drug Abuse (NIDA) began funding clinical studies of ibogaine in the United States in the early 1990s, but terminated the project in 1995.[57] Data demonstrating ibogaine's efficacy in attenuating opioid withdrawal in drug-dependent human subjects was published by Alper et al. in 1999.[58] A cohort of 33 patients were treated with 6 to 29 mg/kg of ibogaine; 25 displayed resolution of the signs of opioid withdrawal from 24 hours to 72 hours post-treatment, but one 24-year-old female, who received the highest dosage, died. Mash et al. (2000), using lower oral doses (10–12 mg/kg) in 27 patients, demonstrated significantly lower objective opiate withdrawal scores in heroin addicts 36 hours after treatment, with self-reports of decreased cocaine and opiate craving and alleviated depression symptoms. Many of these effects appeared sustainable over a one-month post-discharge follow-up.[59]
Society and culture
[edit]Legal status
[edit]As of 2024[update], the legal status of ibogaine varies widely among countries, as it may be illegal to possess or use, may be legalized, may be decriminalized, or is under consideration for future legislation.[60]
In the United States, although some cities and states have decriminalized psychedelic chemicals, plants and mushrooms, ibogaine has had minimal legislation, and remains illegal under federal law, as of 2023.[60][61] The US Drug Enforcement Administration enforces ibogaine as a Schedule I substance under the Controlled Substances Act.[15]
Treatment clinics
[edit]Ibogaine treatment clinics have emerged in Mexico, Bahamas, Canada, the Netherlands, South Africa, and New Zealand, all operating in what has been described as a "legal gray area".[62][63] Costa Rica also has treatment centers.[54] Covert, illegal neighborhood clinics are known to exist in the United States, despite active DEA surveillance.[64] While clinical guidelines for ibogaine-assisted detoxification were released by the Global Ibogaine Therapy Alliance in 2015,[65][66] addiction specialists warn that the treatment of drug dependence with ibogaine in non-medical settings, without expert supervision and unaccompanied by appropriate psychosocial care, can be dangerous — and, in approximately one case in 300, potentially fatal.[63]
Media
[edit]Documentary films
[edit]- Detox or Die (2004). Directed by David Graham Scott. Scott begins videotaping his heroin-addicted friends. Before long, he himself is addicted to the drug. He eventually turns the camera on himself and his family. After 12 years of debilitating, painful dependence on methadone, Scott turns to ibogaine. Filmed in Scotland and England, and broadcast on BBC One as the third installment in the documentary series One Life.[67]
- Ibogaine: Rite of Passage (2004). Directed by Ben Deloenen. Cy, a 34-year-old heroin addict, undergoes ibogaine treatment with Dr. Martin Polanco at the Ibogaine Association, a clinic in Rosarito, Mexico. Deloenen interviews people formerly addicted to heroin, cocaine, and methamphetamine, who share their perspectives about ibogaine treatment. In Gabon, a Babongo woman receives iboga root for her depressive malaise. Deloenen visually contrasts this Western, clinical use of ibogaine with the Bwiti use of iboga root, but emphasizes the Western context.[68]
- Facing the Habit (2007). Directed by Magnolia Martin. Martin's subject is a former millionaire and stockbroker who travels to Mexico for ibogaine treatment for heroin addiction.[69]
- Tripping in Amsterdam (2008). In this short film directed by Jan Bednarz, Simon "Swany" Wan visits Sara Glatt's iboga treatment center in Amsterdam.[70] Current TV broadcast the documentary in 2008 as part of their "Quarter-life Crisis" programming roster.
- I'm Dangerous with Love (2009). Directed by Michel Negroponte. Negroponte examines Dimitri Mobengo Mugianis's long, clandestine career of treating heroin addicts with ibogaine.[71]
- One of the five segments of "Hallucinogens DMT" (2012), Season 2, Episode 4 of Drugs, Inc. on National Geographic Channel, a former heroin user treats addicts with ibogaine in Canada. He himself used ibogaine to stop his abuse of narcotics.[citation needed]
- The "Underground Heroin Clinic" segment of "Addiction" (2013). Season 1, episode 7 of the HBO documentary series Vice examines the use of ibogaine to interrupt heroin addiction.[72][73]
- The Ibogaine Safari (2014). A documentary by filmmaker Pierre le Roux which investigates the claims of painless withdrawal from opiates such as nyaope/heroin in South Africa by taking several addicts on an adventure "safari" while taking ibogaine. The documentary won the award for 'Best Documentary Short' at the 2014 Canada International Film Festival.[74][75]
- Iboga Nights (2014). Directed by David Graham Scott.[76]
- Dosed (2019). A documentary by Tyler Chandler and Nicholas Meyers. Synopsis- After years of no success with prescription drugs, a suicidal Adrianne seeks help from underground healers with her depression, anxiety, and opioid addiction by utilizing illegal psychedelics like magic mushrooms and iboga.[77]
- "Synthetic Ibogaine - Natural Tramadol" (2021). This episode of the documentary series Hamilton's Pharmacopeia on Vice on TV, follows a struggling local addict to an ibogaine ritual.[78]
- Lamar Odom Reborn (2022). A documentary by Mike "Zappy" Zapolin, in which famous NBA athlete (and former Keeping Up With the Kardashians star) Lamar Odom seeks out ibogaine and other therapies to heal PTSD, anxiety, and addiction.[79]
Print media
[edit]While in Wisconsin covering the primary campaign for the United States presidential election of 1972, gonzo journalist Hunter S. Thompson submitted a satirical article to Rolling Stone accusing Democratic Party candidate Edmund Muskie of being addicted to ibogaine. Many readers, and even other journalists, did not realize that the Rolling Stone piece was facetious. The ibogaine assertion, which was completely unfounded, did significant damage to Muskie's reputation, and was cited as a factor in his loss of the nomination to George McGovern.[80] Thompson later said he was surprised that anyone believed it.[81] The article is included in Thompson's post-election anthology, Fear and Loathing on the Campaign Trail '72 (1973).[82]
Author and Yippie Dana Beal co-wrote the 1997 book The Ibogaine Story.[83]
American author Daniel Pinchbeck wrote about his own experience of ibogaine in his book Breaking Open the Head (2002),[84] and in a 2003 article for The Guardian titled "Ten years of therapy in one night".[85]
Author and musician Geoff Rickly based his debut novel Someone Who Isn't Me on his real-life experiences with heroin addiction and an ibogaine clinic in Mexico.[86]
Television drama
[edit]Ibogaine factors into the stories of these episodes from television drama series:
- "Patent Pending". FBI: Most Wanted. Season 4. Episode 6. 15 November 2022. CBS.
- "Via Negativa". The X-Files. Season 8. Episode 7. 17 December 2000. Fox Broadcasting Company.
- "Getting Off". CSI: Crime Scene Investigation. Season 4. Episode 16. 26 February 2004. CBS.
- "Users". Law & Order: Special Victims Unit. Season 11. Episode 7. 4 November 2009. NBC.
- "Echoes". Nikita. Season 1. Episode 16. 24 February 2011. The CW Television Network.
- "One Last Time". Homeland (TV series). Season 3. Episode 9. 24 November 2013. Showtime.
- "Bon Voyage". Graceland (TV series). Season 3. Episode 7. 6 August 2015. USA Network.
Radio
[edit]- "Sink or Swim. Act Two. I'm Not a Doctor But I Play One at the Holiday Inn.". This American Life. Episode 321. 1 December 2006. — A former heroin addict realizes that he wants to help other addicts kick their habits. The problem is, he wants to do this using a hallucinogenic drug - ibogaine - that is completely illegal, and which requires medical expertise he doesn't have.[87]
Research
[edit]Addiction treatment
[edit]The most-studied therapeutic effect of ibogaine is the possible reduction or elimination of addiction to opioids. Research suggests that ibogaine may be useful in treating dependence on other substances such as alcohol, methamphetamine, and nicotine, and may affect compulsive behavioral patterns not involving substance abuse or chemical dependence.[medical citation needed] Researchers note that there remains a "need for systematic investigation in a conventional clinical research setting."[47]
Many users of ibogaine report experiencing visual phenomena during a waking dream state, such as instructive replays of life events that led to their addiction, while others report therapeutic shamanic visions that help them conquer the fears and negative emotions that might drive their addiction. It is proposed that intensive counseling, therapy, and aftercare during the interruption period following treatment is of significant value. Some individuals require a second or third treatment session with ibogaine over the course of 12 to 18 months. A minority of individuals relapse completely into opiate addiction within days or weeks.[88]
Psychotherapy
[edit]Ibogaine was used as an adjunct to psychotherapy by Claudio Naranjo, documented in his book The Healing Journey.[89] He was awarded patent CA 939266 in 1974.
See also
[edit]References
[edit]- ^ a b c d e f Mash DC (April 2023). "IUPHAR - invited review - Ibogaine - A legacy within the current renaissance of psychedelic therapy". Pharmacol Res. 190: 106620. doi:10.1016/j.phrs.2022.106620. PMID 36907284.
- ^ a b c d e f g Maciulaitis R, Kontrimaviciute V, Bressolle FM, Briedis V (March 2008). "Ibogaine, an anti-addictive drug: pharmacology and time to go further in development. A narrative review". Hum Exp Toxicol. 27 (3): 181–194. Bibcode:2008HETox..27..181M. doi:10.1177/0960327107087802. PMID 18650249.
- ^ "Notice - Prescription Drug List (PDL): Multiple additions". Health Canada. Government of Canada, Health. 12 May 2017.
- ^ Galea S, Lorusso M, Newcombe D, Walters C, Williman J, Wheeler A (March 2011). Goodyear-Smith F (ed.). "Ibogaine--be informed before you promote or prescribe" (PDF). Journal of Primary Health Care. 3 (1): 86–7. PMID 21359272. Retrieved 4 December 2015.
- ^ US patent 2813873, Morrice-Marie Janot & Robert Goutarel, "Derivatives of the ibogaine alkaloids", issued 19 November 1957, assigned to Les Laboratoires Gobey
- ^ Soriano-García M (1992). "Structure of ibogaine". Acta Crystallogr. C. 48 (11): 2055–2057. Bibcode:1992AcCrC..48.2055S. doi:10.1107/S0108270192002786.
- ^ Soriano-García M, Walls F, Rodríguez A, Celis IL (1988). "Crystal and molecular structure of ibogamine: An alkaloid from Stemmadenia galeottiana". Journal of Crystallographic and Spectroscopic Research. 18 (2): 197–206. Bibcode:1988JCCry..18..197S. doi:10.1007/BF01181911. S2CID 97519993.
- ^ Arai G, Coppola J, Jeffrey GA (1960). "The structure of ibogaine". Acta Crystallographica. 13 (7): 553–564. Bibcode:1960AcCry..13..553A. doi:10.1107/S0365110X60001369.
- ^ Alper KR, Lotsof HS, Kaplan CD (January 2008). "The ibogaine medical subculture". Journal of Ethnopharmacology. 115 (1): 9–24. doi:10.1016/j.jep.2007.08.034. PMID 18029124. Archived from the original on 6 February 2008.
- ^ a b Alper KR, Beal D, Kaplan CD (2001). "A contemporary history of ibogaine in the United States and Europe" (PDF). The Alkaloids. Chemistry and Biology. 56. Academic Press: 249–81. doi:10.1016/S0099-9598(01)56018-6. ISBN 978-0-12-469556-6. OCLC 119074996. PMID 11705112. Archived from the original (PDF) on 4 March 2016. Retrieved 21 September 2013.
- ^ Obembe SB (2012). Practical Skills and Clinical Management of Alcoholism & Drug Addiction. Elsevier. p. 88. ISBN 9780123985187. OCLC 802345585.
- ^ Brown TK (March 2013). "Ibogaine in the treatment of substance dependence". Current Drug Abuse Reviews. 6 (1): 3–16. doi:10.2174/15672050113109990001. PMID 23627782.
- ^ a b Köck P, Froelich K, Walter M, Lang U, Dürsteler KM (July 2022). "A systematic literature review of clinical trials and therapeutic applications of ibogaine". Journal of Substance Abuse Treatment. 138: 108717. doi:10.1016/j.jsat.2021.108717. PMID 35012793.
- ^ Vastag B (April 2005). "Addiction research. Ibogaine therapy: a 'vast, uncontrolled experiment'". Science. 308 (5720): 345–6. doi:10.1126/science.308.5720.345. PMID 15831735. S2CID 70642078.
- ^ a b c d e f "Iboga (ibogaine)". Drugs.com. 21 April 2023. Retrieved 22 March 2024.
- ^ Alper K, Bai R, Liu N, Fowler SJ, Huang XP, Priori SG, et al. (January 2016). "hERG Blockade by Iboga Alkaloids". Cardiovascular Toxicology. 16 (1): 14–22. doi:10.1007/s12012-015-9311-5. PMID 25636206. S2CID 16071274.
- ^ a b c Koenig X, Hilber K (January 2015). "The anti-addiction drug ibogaine and the heart: a delicate relation". Molecules. 20 (2): 2208–28. doi:10.3390/molecules20022208. PMC 4382526. PMID 25642835.
- ^ O'Hearn E, Molliver ME (July 1993). "Degeneration of Purkinje cells in parasagittal zones of the cerebellar vermis after treatment with ibogaine or harmaline". Neuroscience. 55 (2): 303–10. doi:10.1016/0306-4522(93)90500-f. PMID 8377927. S2CID 25273690.
- ^ a b Mash DC, Kovera CA, Buck BE, Norenberg MD, Shapshak P, Hearn WL, et al. (May 1998). "Medication development of ibogaine as a pharmacotherapy for drug dependence". Annals of the New York Academy of Sciences. 844 (1): 274–92. Bibcode:1998NYASA.844..274M. doi:10.1111/j.1749-6632.1998.tb08242.x. PMID 9668685. S2CID 22068338.
- ^ Alper KR, Stajić M, Gill JR (March 2012). "Fatalities temporally associated with the ingestion of ibogaine". Journal of Forensic Sciences. 57 (2): 398–412. doi:10.1111/j.1556-4029.2011.02008.x. PMID 22268458. S2CID 6670557.
- ^ Glick SD, Maisonneuve IM, Szumlinski KK (2001). "Mechanisms of action of ibogaine: Relevance to putative therapeutic effects and development of a safer iboga alkaloid congener" (PDF). The Alkaloids: Chemistry and Biology. 56: 39–53. doi:10.1016/S0099-9598(01)56006-X. ISBN 978-0-12-469556-6. ISSN 1099-4831. OCLC 119074996. PMID 11705115. Archived from the original (PDF) on 5 April 2014.
- ^ Litjens RP, Brunt TM (2016). "How toxic is ibogaine?". Clinical Toxicology. 54 (4): 297–302. doi:10.3109/15563650.2016.1138226. ISSN 1556-3650. OCLC 6027439727. PMID 26807959. S2CID 7026570.
- ^ Popik P, Skolnick P (1999). "Pharmacology of Ibogaine and Ibogaine-Related Alkaloids". The Alkaloids: Chemistry and Biology. 52. Academic Press: 197–231. doi:10.1016/s0099-9598(08)60027-9. ISBN 978-0-12-469552-8. ISSN 1099-4831. OCLC 505140539. Archived from the original on 26 May 2012.
- ^ Alper KR (2001). Alper KR, Glick SD (eds.). "Ibogaine: A Review" (PDF). The Alkaloids: Chemistry and Biology. 56. San Diego: Academic: 1–38. doi:10.1016/S0099-9598(01)56005-8. ISBN 978-0-12-469556-6. ISSN 1099-4831. OCLC 119074989. PMID 11705103. Archived from the original (PDF) on 27 September 2007.
- ^ a b Maillet EL, Milon N, Heghinian MD, Fishback J, Schürer SC, Garamszegi N, et al. (December 2015). "Noribogaine is a G-protein biased κ-opioid receptor agonist". Neuropharmacology. 99 (December 2015): 675–88. doi:10.1016/j.neuropharm.2015.08.032. ISSN 0028-3908. OCLC 5921346571. PMID 26302653.
- ^ Antonio T, Childers SR, Rothman RB, Dersch CM, King C, Kuehne M, et al. (2013). "Effect of Iboga alkaloids on µ-opioid receptor-coupled G protein activation". PLOS ONE. 8 (10): e77262. Bibcode:2013PLoSO...877262A. doi:10.1371/journal.pone.0077262. ISSN 1932-6203. OCLC 5534534188. PMC 3818563. PMID 24204784.
- ^ Zubaran C, Shoaib M, Stolerman IP, Pablo J, Mash DC (July 1999). "Noribogaine generalization to the ibogaine stimulus: correlation with noribogaine concentration in rat brain". Neuropsychopharmacology. 21 (1): 119–26. doi:10.1016/S0893-133X(99)00003-2. ISSN 1740-634X. OCLC 9523107895. PMID 10379526.
- ^ Baumann MH, Rothman RB, Pablo JP, Mash DC (May 2001). "In vivo neurobiological effects of ibogaine and its O-desmethyl metabolite, 12-hydroxyibogamine (noribogaine), in rats". The Journal of Pharmacology and Experimental Therapeutics. 297 (2): 531–9. ISSN 0022-3565. OCLC 118935157. PMID 11303040.
- ^ Hough LB, Bagal AA, Glick SD (March 2000). "Pharmacokinetic characterization of the indole alkaloid ibogaine in rats". Methods and Findings in Experimental and Clinical Pharmacology. 22 (2): 77–81. doi:10.1358/mf.2000.22.2.796066. ISSN 0379-0355. OCLC 118905358. PMID 10849889.
- ^ Mash DC, Kovera CA, Pablo J, Tyndale RF, Ervin FD, Williams IC, et al. (September 2000). "Ibogaine: complex pharmacokinetics, concerns for safety, and preliminary efficacy measures". Annals of the New York Academy of Sciences. 914 (1): 394–401. Bibcode:2000NYASA.914..394M. CiteSeerX 10.1.1.598.8242. doi:10.1111/j.1749-6632.2000.tb05213.x. ISSN 0077-8923. OCLC 117596065. PMID 11085338. S2CID 33436971.
- ^ Shulgin A, Shulgin A (1997). "IBOGAINE; 12-METHOXYIBOGAMINE". Tihkal: the continuation. Berkeley, CA: Transform Press. pp. 487–490. ISBN 978-0-9630096-9-2. OCLC 1360062118 – via Internet Archive.
- ^ Jana GK, Sinha S (2012). "Total synthesis of ibogaine, epiibogaine and their analogues". Tetrahedron. 68 (35): 7155–7165. doi:10.1016/j.tet.2012.06.027. ISSN 0040-4020. OCLC 5901603422.
- ^ a b Jenks CW (February 2002). "Extraction studies of Tabernanthe iboga and Voacanga africana". Natural Product Letters. 16 (1): 71–6. doi:10.1080/1057563029001/4881. ISSN 1057-5634. OCLC 4803437833. PMID 11942686. S2CID 23390825.
- ^ "Voacanga Extraction Manual: Phase 4: Production and Purification of Ibogaine" (PDF). www.puzzlepiece.org. Retrieved 4 December 2015.
- ^ Krengel F, Mijangos MV, Reyes-Lezama M, Reyes-Chilpa R (July 2019). "Extraction and Conversion Studies of the Antiaddictive Alkaloids Coronaridine, Ibogamine, Voacangine, and Ibogaine from Two Mexican Tabernaemontana Species (Apocynaceae)". Chemistry & Biodiversity. 16 (7): e1900175. doi:10.1002/cbdv.201900175. ISSN 1612-1872. OCLC 8185274820. PMID 31095891. S2CID 157058497.
- ^ Pace CJ, Glick SD, Maisonneuve IM, He LW, Jokiel PA, Kuehne ME, et al. (May 2004). "Novel iboga alkaloid congeners block nicotinic receptors and reduce drug self-administration". European Journal of Pharmacology. 492 (2–3): 159–67. doi:10.1016/j.ejphar.2004.03.062. ISSN 0014-2999. OCLC 110898054. PMID 15178360.
- ^ Glick SD, Kuehne ME, Raucci J, Wilson TE, Larson D, Keller RW, et al. (September 1994). "Effects of iboga alkaloids on morphine and cocaine self-administration in rats: relationship to tremorigenic effects and to effects on dopamine release in nucleus accumbens and striatum". Brain Research. 657 (1–2): 14–22. doi:10.1016/0006-8993(94)90948-2. ISSN 0006-8993. OCLC 4923262393. PMID 7820611. S2CID 1940631.
- ^ Hua T (28 January 2006). "Antiaddictive indole alkaloids in Ervatamia yunnanensis and their bioactivity". Academic Journal of Second Military Medical University. Archived from the original on 13 February 2012. Retrieved 15 August 2012.
- ^ Cameron LP, Tombari RJ, Lu J, Pell AJ, Hurley ZQ, Ehinger Y, et al. (January 2021). "A non-hallucinogenic psychedelic analogue with therapeutic potential". Nature. 589 (7842): 474–479. Bibcode:2021Natur.589..474C. doi:10.1038/s41586-020-3008-z. ISSN 0028-0836. OCLC 8816084004. PMC 7874389. PMID 33299186.
- ^ Hamilton J (9 December 2020). "Progress Toward A Safer Psychedelic Drug To Treat Depression And Addiction". NPR.org. Retrieved 12 December 2020.
- ^ Iyer RN, Favela D, Zhang G, Olson DE (March 2021). "The iboga enigma: the chemistry and neuropharmacology of iboga alkaloids and related analogs". Natural Product Reports. 38 (2): 307–329. doi:10.1039/D0NP00033G. ISSN 0265-0568. OCLC 8646253022. PMC 7882011. PMID 32794540.
- ^ "Marie Théophile GRIFFON du BELLAY (1829–1908)". ecole.nav.traditions.free.fr. 5 February 2023. Archived from the original on 6 March 2023.
- ^ Dybowski J, Landrin E (1901). "PLANT CHEMISTRY. Concerning Iboga, its excitement-producing properties, its composition, and the new alkaloid it contains, ibogaine". C. R. Acad. Sci. 133: 748. Archived from the original on 10 July 2013. Retrieved 20 May 2013.
- ^ Büchi G, Coffen DL, Kocsis K, Sonnet PE, Ziegler FE (1966). "The Total Synthesis of Iboga Alkaloids". J. Am. Chem. Soc. 88 (13): 3099–3109. Bibcode:1966JAChS..88.3099B. doi:10.1021/ja00965a039.
- ^ Frauenfelder C (1999). Ph.D. (Thesis). p. 24. Archived from the original (PDF) on 29 July 2012.
- ^ Freedlander J (2003). "Ibogaine: a Comprehensive Literature Review". Journal of Drug Addiction, Education, and Eradication. 1. Nova Science Publishers: 79–98. ISSN 1546-6965. OCLC 609715451. Retrieved 23 July 2024 – via ibogaine.mindvox.com.
- ^ a b Alper KR, Lotsof HS, Frenken GM, Luciano DJ, Bastiaans J (1999). "Treatment of acute opioid withdrawal with ibogaine" (PDF). The American Journal on Addictions. 8 (3): 234–42. doi:10.1080/105504999305848. ISSN 1055-0496. OCLC 122057314. PMID 10506904. Archived from the original (PDF) on 26 January 2002.
- ^ Hevesi D (17 February 2010). "Howard Lotsof Dies at 66; Saw Drug Cure in a Plant". The New York Times. ISSN 0362-4331. Retrieved 11 June 2015.
- ^ Dzoljic ED, Kaplan CD, Dzoljic MR (1988). "Effect of ibogaine on naloxone-precipitated withdrawal syndrome in chronic morphine-dependent rats". Archives Internationales de Pharmacodynamie et de Therapie. 294: 64–70. ISSN 0003-9780. OCLC 115924585. PMID 3233054.
- ^ Glick SD, Rossman K, Steindorf S, Maisonneuve IM, Carlson JN (April 1991). "Effects and aftereffects of ibogaine on morphine self-administration in rats". European Journal of Pharmacology. 195 (3): 341–5. doi:10.1016/0014-2999(91)90474-5. ISSN 0014-2999. OCLC 121337019. PMID 1868880.
- ^ Cappendijk SL, Dzoljic MR (September 1993). "Inhibitory effects of ibogaine on cocaine self-administration in rats". European Journal of Pharmacology. 241 (2–3): 261–5. doi:10.1016/0014-2999(93)90212-Z. ISSN 0014-2999. OCLC 121698805. PMID 8243561.
- ^ Rezvani AH, Overstreet DH, Lee YW (November 1995). "Attenuation of alcohol intake by ibogaine in three strains of alcohol-preferring rats". Pharmacology, Biochemistry, and Behavior. 52 (3): 615–20. doi:10.1016/0091-3057(95)00152-M. ISSN 0091-3057. OCLC 121166985. PMID 8545483. S2CID 38567079.
- ^ Ditton MC (17 July 2007). "A Home for Ibogaine in Barcelona". The Huffington Post. Retrieved 11 June 2015.
- ^ a b "Costa Rican Center a Leader in Alternative Ibogaine Therapy". Treatment Magazine. 11 September 2012. Archived from the original on 3 December 2013. Retrieved 11 June 2015.
- ^ Kontrimaviciūte V, Mathieu O, Mathieu-Daudé JC, Vainauskas P, Casper T, Baccino E, et al. (September 2006). "Distribution of ibogaine and noribogaine in a man following a poisoning involving root bark of the Tabernanthe iboga shrub". Journal of Analytical Toxicology. 30 (7): 434–40. doi:10.1093/jat/30.7.434. ISSN 0146-4760. OCLC 5113088519. PMID 16959135.
- ^ Taylor WI (1965). "Chapter 9 The Iboga and Voacanga Alkaloids" (PDF). The Alkaloids: Chemistry and Physiology. Vol. 8. Elsevier. pp. 203–235. doi:10.1016/s1876-0813(08)60048-2. ISBN 978-0-12-469508-5. OCLC 4922128763 – via Puzzle Piece.
- ^ Doblin R (12 May 2003). "A Non-Profit Approach to Developing Ibogaine into an FDA-Approved Medication". Archived from the original (PPT) on 30 October 2012.
- ^ Alper KR, Lotsof HS, Frenken GM, Luciano DJ, Bastiaans J (1999). "Treatment of Acute Opioid Withdrawal with Ibogaine" (PDF). The American Journal on Addictions. 8 (3): 234–242. doi:10.1080/105504999305848. ISSN 1055-0496. OCLC 122057314. PMID 10506904. Archived from the original (PDF) on 26 January 2002.
- ^ Mash DC, Kovera CA, Pablo J, Tyndale RF, Ervin FD, Williams IC, et al. (September 2000). "Ibogaine: Complex Pharmacokinetics, Concerns for Safety, and Preliminary Efficacy Measures" (PDF). Annals of the New York Academy of Sciences. 914 (1): 394–401. Bibcode:2000NYASA.914..394M. CiteSeerX 10.1.1.598.8242. doi:10.1111/j.1749-6632.2000.tb05213.x. ISSN 0077-8923. OCLC 117596065. PMID 11085338. S2CID 33436971. Archived from the original (PDF) on 3 March 2007. Retrieved 6 July 2006.
- ^ a b Chesak J (22 March 2024). "What psychedelics legalisation and decriminalisation looks like around the world". BBC. Retrieved 22 March 2024.
- ^ Siegel JS, Daily JE, Perry DA, Nicol GE (January 2023). "Psychedelic Drug Legislative Reform and Legalization in the United States". JAMA Psychiatry. 80 (1): 77–83. doi:10.1001/jamapsychiatry.2022.4101. ISSN 2168-622X. OCLC 9705254062. PMC 10069558. PMID 36477830.
- ^ Hegarty S (10 April 2012). "Can a hallucinogen from Africa cure addiction?". BBC News. Retrieved 24 July 2024.
- ^ a b "Ibogaine Therapy". Multidisciplinary Association for Psychedelic Studies - MAPS. 19 April 1979. Retrieved 24 July 2024.
- ^ Hunter A (13 December 2005). "Busted for Iboga". The Village Voice. Retrieved 24 July 2024.
- ^ >"Clinical Guidelines for Ibogaine-Assisted Detoxification". IBOGAINE SAFETY GUIDELINES. 28 October 2020. Retrieved 24 July 2024.
- ^ "The Global Ibogaine Therapy Alliance (GITA)". The Global Ibogaine Therapy Alliance. Archived from the original on 25 January 2016. Retrieved 14 January 2016.
- ^ "Detox or Die". BFI. One Life. 8 June 2004. Retrieved 24 July 2024.
- ^ "Ibogaine: Rite of Passage". IFFR EN. January–February 2005. Retrieved 2 September 2024.
- ^ "The 2007 FFF Winners". San Francisco Frozen Film Festival (SFFFF). 13 July 2007. Facing the Habit (WORLD PREMIERE). Retrieved 2 September 2024.
- ^ "Tripping in Amsterdam". Current.com. 23 June 2008. Archived from the original on 11 October 2012. Retrieved 7 April 2013.
- ^ Lanthier JJ (10 January 2011). "Review: I'm Dangerous with Love". Slant Magazine. Retrieved 2 September 2024.
- ^ "A New Episode of Our TV Show Is Airing Tonight". VICE. 17 May 2013. Retrieved 9 September 2024.[non-primary source needed]
- ^ Tobaccoland & Underground Heroin Clinic | VICE on HBO (Season 1, Episode 7) on YouTube
- ^ "Ibogaine Media". ibogaworld.com. 13 November 2019. Retrieved 9 September 2024.[better source needed]
- ^ The Ibogaine Safari on YouTube
- ^ "Iboga Nights". Top Documentary Films. 31 July 2015. Retrieved 4 September 2024.
- ^ Kenigsberg B (19 March 2020). "'Dosed' Review: The Case for Plant-Based Recovery". The New York Times. Retrieved 6 September 2024.
- ^ Phelps H (21 June 2021). "Hulu's 'Hamilton's Pharmacopeia' Shows That We Can No Longer Ignore Connections Between Religion and Drugs". Religion Dispatches. Retrieved 8 September 2024.
- ^ Hernandez V (10 June 2021). "Lamar Odom prepares to fight Aaron Carter, but first he fought PTSD". Los Angeles Times. Retrieved 9 September 2024.
- ^ "Online NewsHour: Remembering Ed Muskie". pbs.org. 3 February 1999. Archived from the original on 27 April 1999. Retrieved 17 January 2023.
- ^ Gibney A (2008). Gonzo: The Life and Work of Dr. Hunter S. Thompson (Motion picture). OCLC 259718859.
- ^ Fear and Loathing on the Campaign Trail '72. Straight Arrow Books. 1973. pp. 150–54. ISBN 978-0-87932-053-9. OCLC 636410.
- ^ Beal D, De Rienzo P (1997). The Ibogaine Story: Report on the Staten Island Project. Autonomedia. ISBN 1570270295.
- ^ Pinchbeck D (2002). Breaking Open the Head: A Psychedelic Journey into the Heart of Contemporary Shamanism. Broadway Books. ISBN 9780767907422. OCLC 50601753.
- ^ Pinchbeck D (19 September 2003). "Ten years of therapy in one night". The Guardian. London. Retrieved 15 December 2013.
- ^ Rickly G. Someone Who Isn't Me.
- ^ Olkowski L, Kay T (1 December 2006). "Sink or Swim. Act Two. I'm Not A Doctor But I Play One At The Holiday Inn.". This American Life. Episode 321. 16 minutes in. Retrieved 17 August 2015.
- ^ Lotsof HS (1995). "Ibogaine in the Treatment of Chemical Dependence Disorders: Clinical Perspectives". MAPS Bulletin. 3: 19–26. Archived from the original on 22 January 1997.
- ^ Naranjo C (1973). "V, Ibogaine: Fantasy and Reality". The healing journey: new approaches to consciousness. New York: Pantheon Books. pp. 197–231. ISBN 978-0-394-48826-4. Retrieved 15 August 2012.
External links
[edit]- "News on ibogaine". National Center for Biotechnical Information. 19 March 2020. Retrieved 6 September 2024.
- Drugs not assigned an ATC code
- Alkaloids found in Apocynaceae
- Alkaloids found in Iboga
- Dream
- Drug rehabilitation
- Entheogens
- Experimental hallucinogens
- HERG blocker
- Indole alkaloids
- Nicotinic antagonists
- NMDA receptor antagonists
- Oneirogens
- Phenol ethers
- Plant toxins
- Serotonin receptor agonists
- Sigma agonists
- Tryptamines
- VMAT inhibitors