Glyphosate: Difference between revisions
m Dating maintenance tags: {{Relevance-inline}} {{Clarification needed}} |
→Toxicity: That source actually says "Although surfactants probably contribute to the acute toxicity of glyphosate formulations, the weight of evidence is against surfactants potentiating the toxicity of glyphosate." - the precise opposite of what's c |
||
| Line 144: | Line 144: | ||
== Toxicity == |
== Toxicity == |
||
Glyphosate is the active ingredient in herbicide formulations containing it. However, in addition to glyphosate salts, commercial formulations of glyphosate contain additives such as [[surfactant]]s which vary in nature and concentration. |
Glyphosate is the active ingredient in herbicide formulations containing it. However, in addition to glyphosate salts, commercial formulations of glyphosate contain additives such as [[surfactant]]s which vary in nature and concentration. Toxicologists have studied glyphosate alone, additives alone, and formulations. |
||
=== Active ingredient === |
=== Active ingredient === |
||
Revision as of 15:03, 5 October 2015

| |

| |

| |
| Names | |
|---|---|
| IUPAC name
N-(phosphonomethyl)glycine
| |
| Other names
2-[(phosphonomethyl)amino]acetic acid
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.012.726 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H8NO5P | |
| Molar mass | 169.073 g·mol−1 |
| Appearance | white crystalline powder |
| Density | 1.704 (20 °C) |
| Melting point | 184.5 °C (364.1 °F; 457.6 K) |
| Boiling point | decomposes at 187 °C (369 °F; 460 K) |
| 1.01 g/100 mL (20 °C) | |
| log P | −2.8 |
| Acidity (pKa) | <2, 2.6, 5.6, 10.6 |
| Hazards | |
| GHS labelling: | |
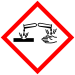 
| |
| Danger | |
| H318, H411 | |
| P273, P280, P305+P351+P338, P310, P501 | |
| Flash point | Non-flammable |
| Safety data sheet (SDS) | InChem MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Glyphosate (N-(phosphonomethyl)glycine) is a broad-spectrum systemic herbicide used to kill weeds, especially annual broadleaf weeds and grasses known to compete with commercial crops grown around the globe. It was discovered to be an herbicide by Monsanto chemist John E. Franz in 1970.[3] Monsanto brought it to market in the 1970s under the trade name Roundup and Monsanto's last commercially relevant United States patent expired in 2000.
Glyphosate was quickly adopted by farmers, even more so when Monsanto introduced glyphosate-resistant crops, enabling farmers to kill weeds without killing their crops. In 2007, glyphosate was the most used herbicide in the United States agricultural sector, with 180 to 185 million pounds (82,000 to 84,000 tonnes) applied, and the second-most used in home and garden market where users applied 5 to 8 million pounds (2,300 to 3,600 tonnes); in addition, industry, commerce, and government applied 13 to 15 million pounds (5,900 to 6,800 tonnes).[4]
With its heavy use in agriculture, weed resistance to glyphosate is a growing problem. While glyphosate and formulations such as Roundup have been approved by regulatory bodies worldwide and are widely used, concerns about their effects on humans and the environment persist.[5]
Glyphosate's mode of action as an herbicide is to inhibit a plant enzyme involved in the synthesis of the aromatic amino acids: tyrosine, tryptophan, and phenylalanine. It is absorbed through foliage, and minimally through roots,[6][7][8] and translocated to growing points. Because of this mode of action, it is only effective on actively growing plants; it is not effective as a pre-emergence herbicide. Some crops have been genetically engineered to be tolerant of glyphosate (i.e., Roundup Ready, also created by Monsanto Company). Such crops allow farmers to use glyphosate as a postemergence herbicide against weeds, but the development of glyphosate resistance in some weed species is emerging as a costly problem. Roundup Ready soybean was the first Roundup Ready crop.
Many regulatory and scholarly reviews have evaluated the relative toxicity of glyphosate as an herbicide. The German Federal Institute for Risk Assessment published a toxicology review in 2013, which found that "the available data is contradictory and far from being convincing" with regard to correlations between exposure to glyphosate formulations and risk of various cancers, including non-Hodgkin lymphoma (NHL).[9] A meta-analysis published in 2014 identified an increased risk of NHL in workers exposed to glyphosate formulations.[10] In March 2015 the World Health Organization's International Agency for Research on Cancer published a summary of its forthcoming monograph on glyphosate, and classified it as "probably carcinogenic in humans" (category 2A) based on epidemiological studies, animal studies, and in vitro studies.[5][11][12]
Discovery
Glyphosate was first synthesized in 1950 by Swiss chemist Henry Martin, who worked for the Swiss company Cilag. The work was never published.[13]: 1 Stauffer Chemical patented the agent as a chemical chelator in 1964 as it binds and removes minerals such as calcium, magnesium, manganese, copper and zinc.[14]
Somewhat later, glyphosate was independently discovered at Monsanto in 1970. Monsanto chemists had synthesized about 100 analogs of aminomethylphosphonic acid as potential water-softening agents. Two were found to have weak herbicidal activity, and John E. Franz, a chemist at Monsanto, was asked to try to make analogs with stronger herbicidal activity. Glyphosate was the third analog he made.[13]: 1–2 [15]
Glyphosate has been called by experts in herbicides "virtually ideal" due to its broad spectrum and low toxicity to animal life compared with other herbicides.[16][17][18][19] Franz received the National Medal of Technology in 1987[20] and the Perkin Medal for Applied Chemistry[21] in 1990 for his discoveries. Franz was inducted into the National Inventor's Hall of Fame in 2007.[22]
Chemistry

Glyphosate is an aminophosphonic analogue of the natural amino acid glycine, and the name is a contraction of gly(cine) phos(phon)ate. The molecule has several dissociable hydrogens, especially the first hydrogen of the phosphate group. The molecule tends to exist as a zwitterion where a phosphonic hydrogen dissociates and joins the amine group. Glyphosate is soluble in water to 12 g/L at room temperature.

The main deactivation path is hydrolysis to aminomethylphosphonic acid (AMPA).[23]
Biochemistry
Glyphosate kills plants and many bacteria by interfering with the synthesis of the aromatic amino acids phenylalanine, tyrosine, and tryptophan. It does this by inhibiting the enzyme 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS), which catalyzes the reaction of shikimate-3-phosphate (S3P) and phosphoenolpyruvate to form 5-enolpyruvyl-shikimate-3-phosphate (EPSP).[24]
EPSP is subsequently dephosphorylated to chorismate, an essential precursor for the amino acids mentioned above.[25] These amino acids are used in protein synthesis and to produce secondary metabolites such as folates, ubiquinones, and naphthoquinone.
X-ray crystallographic studies of glyphosate and EPSPS show that glyphosate functions by occupying the binding site of the phosphoenolpyruvate, mimicking an intermediate state of the ternary enzyme substrates complex.[26][27] Glyphosate inhibits the EPSPS enzymes of different species of plants and microbes at different rates.[28][29]
EPSPS is produced only by plants and microbes; the gene coding for it is not in the mammalian genome.[30][31] Gut flora of some animals contain EPSPS.[32]
Glyphosate is absorbed through foliage and minimally through roots. Because of this mode of action, it is only effective on actively growing plants; it is not effective in preventing seeds from germinating.[7][8]
Environmental fate
Glyphosate adsorbs strongly to soil, and residues are expected to generally be immobile in soil. Ground and surface water pollution is limited. Glyphosate is readily degraded by soil microbes to aminomethylphosphonic acid (AMPA). Glyphosate and AMPA are not likely to move to ground water due to their strong adsorptive characteristics. Glyphosate does have the potential to contaminate surface waters due to its aquatic use patterns and through erosion, as it adsorbs to soil particles suspended in runoff. Limited leaching can also occur after high rainfall after application. If glyphosate reaches surface water, it is not broken down readily by water or sunlight.[33][34]
The half-life of glyphosate in soil ranges between 2 and 197 days; a typical field half-life of 47 days has been suggested. Soil and climate conditions affect glyphosate's persistence in soil. The median half-life of glyphosate in wate varies from a few to 91 days.[7] At a site in Texas half-live was as little as three days to 141 days at a site in Iowa.[35] The glyphosate metabolite AMPA has been found in Swedish forest soils up to two years after a glyphosate application. In this case, the persistence of AMPA was attributed to the soil being frozen for most of the year.[36] Glyphosate adsorption to soil, and later release from soil, varies depending on the kind of soil.[37][38] A 2009 study using a RoundUp formulation concluded absorption into plants delays subsequent soil degradation and can increase glyphosate persistence in soil from two to six times.[39]
According to the National Pesticide Information Center fact sheet, glyphosate is not included in compounds tested for by the Food and Drug Administration's Pesticide Residue Monitoring Program, nor in the United States Department of Agriculture's Pesticide Data Program. However, a field test showed that lettuce, carrots, and barley contained glyphosate residues up to one year after the soil was treated with 3.71 lb of glyphosate per acre (4.15 kg per hectare).[7]
Use

Glyphosate is effective in killing a wide variety of plants, including grasses and broadleaf and woody plants. By volume, it is one of the most widely used herbicides.[7] It is commonly used for agriculture, horticulture, viticulture, and silviculture purposes, as well as garden maintenance (including home use).[7][40] It has a relatively small effect on some clover species and morning glory.[41]
In many cities, glyphosate is sprayed along the sidewalks and streets, as well as crevices in between pavement where weeds often grow. However, up to 24% of glyphosate applied to hard surfaces can be run off by water.[42] Glyphosate contamination of surface water is attributed to urban and agricultural use.[43] Glyphosate is used to clear railroad tracks and get rid of unwanted aquatic vegetation.[8] Since 1994, glyphosate has been used in aerial spraying in Colombia in coca eradication programs; Colombia announced in May 2015 that by October it would cease using glyphosate in these programs due to concerns about human toxicity of the chemical.[44]
In addition to its use as an herbicide, glyphosate is also used for crop desiccation (siccation) to increase harvest yield[8] and, as a result of desiccation, to increase sucrose concentration in sugarcane before harvest.[45]
Formulations and tradenames
Glyphosate is marketed in the United States and worldwide by many agrochemical companies, in different solution strengths and with various adjuvants, under dozens of tradenames.[46][47][48][49] As of 2010, there were more than 750 glyophosate products on the market.[50] In 2012, in terms of volume about half of the total global consumption of glyphosate was for conventional crops; Asia Pacific was the largest and fastest growing market.[51] Chinese manufacturers collectively are the world's largest producers of glyphosate and its precursors[52] and account for about 30% of global exports.[51] Key manufacturers include Anhui Huaxing Chemical Industry Compan, BASF, Bayer CropScience, Dow AgroSciences, DuPont, Jiangsu Good Harvest-Weien Agrochemical Company, Monsanto, Nantong Jiangshan Agrochemical & Chemicals Co., Nufarm Limited, SinoHarvest, Syngenta, and Zhejiang Xinan Chemical Industrial Group Company.[51]
Glyphosate is an acid molecule, so it is formulated as a salt for packaging and handling. Various salt formulations include isopropylamine, diammonium, monoammonium, or potassium as the counterion. Some brands include more than one salt. Some companies report their product as acid equivalent (ae) of glyphosate acid, or some report it as active ingredient (ai) of glyphosate plus the salt, and others report both. To compare performance of different formulations, knowledge of how the products were formulated is needed. Since the salt does not contribute to weed control and different salts have different weights, the acid equivalent is a more accurate method of expressing and comparing concentrations.[53] Adjuvant loading refers to the amount of adjuvant[54][55] already added to the glyphosate product. Fully loaded products contain all the necessary adjuvants, including surfactant; some contain no adjuvant system, while other products contain only a limited amount of adjuvant (minimal or partial loading) and additional surfactants must be added to the spray tank before application.[53] As of 2000 (just before Monsanto's patent on glyphosate expired), over 400 commercial adjuvants from over 34 different companies were available for use in commercial agriculture.[56][57]
Products are supplied most commonly in formulations of 120, 240, 360, 480, and 680 g/l of active ingredient. The most common formulation in agriculture is 360 g/l, either alone or with added cationic surfactants.[47]
For 360 g/l formulations, European regulations allow applications of up to 12 l/ha for control of perennial weeds such as couch grass. More commonly, rates of 3 l/ha are practiced for control of annual weeds between crops.[58]
Monsanto
Monsanto developed and patented the use of glyphosate to kill weeds in the 1970s, and has marketed it as Roundup since 1973. It retained exclusive rights in the United States until its patent expired in September, 2000.
As of 2009, sales of these herbicide products represented about 10% of Monsanto's revenue due to competition from other producers of other glyphosate-based herbicides;[59] their Roundup products (which include GM seeds) represented about half of Monsanto's gross margin.[60]
The active ingredient of the Monsanto herbicides is the isopropylamine salt of glyphosate. Another important ingredient in some formulations is the surfactant polyethoxylated tallow amine.
Monsanto also produces seeds which grow into plants genetically engineered to be tolerant to glyphosate. The genes contained in these seeds are patented. Such crops allow farmers to use glyphosate as a postemergence herbicide against most broadleaf and cereal weeds. Soy was the first glyphosate-resistant crop.
Toxicity
Glyphosate is the active ingredient in herbicide formulations containing it. However, in addition to glyphosate salts, commercial formulations of glyphosate contain additives such as surfactants which vary in nature and concentration. Toxicologists have studied glyphosate alone, additives alone, and formulations.
Active ingredient
Mammals
The LD50 of glyphosate is 5,000 mg/kg for rats, 10,000 mg/kg in mice and 3,530 mg/kg in goats. The acute dermal LD50 in rabbits is greater than 2 g/kg. Mammalian LD50s are considered to be low to very low toxicity. Signs of glyphosate toxicity in animals typically appear within 30 minutes to 2 hours following ingestion of a large enough dose, and include initial excitability and tachycardia, ataxia, depression and bradycardia but severe cases can develop into collapse and convulsions.[7]
A review of unpublished short-term rabbit feeding studies reported severe toxicity effects at 150 mg/kg/day and "no observed adverse effect level" (NOAEL) doses ranging from 50 to 200 mg/kg/day.[61]
Glyphosate can have carcinogenic effects in non-human mammals. These include the induction of positive trends in the incidence of renal tubule carcinoma and haemangiosarcoma in male mice, and increased pancreatic islet-cell adenoma in male rats.[11] A 2015 review found that glyphosate may be toxic below the lowest-observed-adverse-effect level that has been assigned to it by regulators, and that its effects may include "teratogenic, tumorigenic and hepatorenal effects."[62]
Humans
Human acute toxicity is dose-related. Acute fatal toxicity has been reported in deliberate overdose.[63][64] Early epidemiological studies did not find associations between long-term low-level exposure to glyphosate and any disease.[65][66][67] In 2013 the European commission reviewed a 2002 finding that had concluded equivocal evidence existed of a relationship between glyphosate exposure during pregnancy and cardiovascular malformations and found that "there is no increased risk at the levels of exposure below those that caused maternal toxicity."[68] A 2013 review found that neither glyphosate nor typical glyphosate-based formulations (GBFs) pose a genotoxicity risk in humans under normal conditions of human or environmental exposures.[69] A 2000 review concluded that "under present and expected conditions of new use, there is no potential for Roundup herbicide to pose a health risk to humans".[70] A 2002 review by the European Union reached the same conclusion.[71] A 2014 review article reported a significant association between B-cell lymphoma and glyphosate exposure.[10]
Fish and aquatic life
Glyphosate is generally less persistent in water than in soil, with 12 to 60-day persistence observed in Canadian ponds, yet persistence of over a year has been recorded in the sediments of U.S. ponds.[33] Low glyphosate concentrations can be found in many creeks and rivers in the U.S. and in Europe.[72] The half-life of glyphosate in water is between 12 days to 10 weeks.[73]
In various freshwater fish species, pure glyphosate has a 48-hour lethal concentration (LC50) of greater than 24 mg/L to 140 mg/L, while marketed glyphosate formulations can range from 1.3 mg/L to greater than 1000 mg/L. Specific species LC50s include 140 mg/L for rainbow trout (Onchorynchus mykiss), 97 mg/L for fathead minnows (Pimephales promelas), 130 mg/L for channel catfish (Icalurus punctatus) and 150 mg/L for bluegill sunfish (Lepomis macrochirus). A positive association between exposure to glyphosate and immunotoxicity in fish has been reported and other studies have consistently shown that glyphosate can cause oxidative stress in fish.[74]
For green frogs (Rana clamitans) glyphosate has a 24 and 96-hour LC50 of greater than 38.9 g/L. A 2014 review found that amphibians have been identified as particularly sensitive to glyphosate formulations in their larval and tadpole stages of development, and toxicity has been extensively studied for those stages; toxicity for terrestrial life cycle stages is less well understood.[75] The review found that "across the spectrum of organisms likely to be exposed to glyphosate in the aquatic environment, it has been shown that sensitivity to glyphosate (and the constituents of commercial formulas) is highly species-specific. A 2012 study on the toxic potential of environmentally relevant concentrations of glyphosate on wood frogs (Rana sylvatica), leopard frogs (Rana pipiens pipiens) and American toads (Bufo americanus) reported a significant induction of morphological changes in the tadpoles of the three species, including alteration of the size of the tadpole tail. This indicates that the herbicide could be affecting the mechanisms of development that are normally used as defence responses against predators. These results showed that glyphosate "can have widespread and relevant effects on non target species."[76] Developmental toxicity appears to occur in the larval and tadpoles of the American toad at levels of exposure to glyphosate that occur in common use of the herbicide.[75]
In freshwater invertebrates (species unspecified), glyphosate has a 48-hour LC50 ranging from 55 to 780 ppm. The 96-hour LC50 is 281 ppm for grass shrimps (Palaemonetas vulgaris) and 934 ppm for fiddler crabs (Uca pagilator). These values make glyphosate "slightly toxic to practically non-toxic".[7]
A study observing the impact of herbicides on the biodiversity of aquatic communities containing algae and more than 25 species of animals showed that in contrast to 2,4-D, glyphosate had great impact in the community, causing a decrease of 22% of the species richness.[76]
Antimicrobial activity
The antimicrobial activity of glyphosate has been described in the microbiology literature since its discovery in 1970 and the description of glyphosate's mechanism of action in 1972. Efficacy was described for numerous bacterial and fungi.[77] In 1998 Monsanto discovered efficacy against apicomplexan parasites.[78] filing a patent in 2003 and in 2010 receiving the patent for glyphosate in conjunction with oxalic acid as treatment for parasitic infections in mammals, including Toxoplasma gondii, Plasmodium falciparum and Cryptosporidium parvum.
The singular antimicrobial effects are: Inhibition of gram negative bacteria like Enterobacteriaceae, and gram positives, like Enterococcus, each with a different pattern of inhibition,[79] mycobacteriaceae. Inhibition of some Rhizobium species, especially under moisture stress (Bradyrhizobium japonicum which is important for soybean nitrogen fixation)[80] relative resistance of Cryptococcus neoformans[81]
- Microbiome, dairy cows
The rumen of nearly 260 dairy cows was examined in correlation to their glyphosate exposure as measured level in urine. Two parameters of the innate immune system were decreased and the fungal community changed.[82] Enterococcus bacteria are part of the regular gut flora which keep the bacterium Clostridium botulinum in check; Glyphosate is exquisitely toxic to Enterocci but not Clostridia and may be "important in the understanding of what is termed chronic botulism in farm animals".[83]
- Microbes, soil
Glyphosate increased the growth of the mold Aspergillus niger in soil.[84]
Soil biota

When glyphosate comes into contact with the soil, it can be bound to soil particles, thereby slowing its degradation.[33][35] Farmers have noticed changes in soil texture, more compaction, less root nodules in corn.[86] In sewage sludge, unbound glyphosate has been thought to be degraded by bacteria.[87][relevant?] Glyphosate and its degradation product, AMPA, residues are considered to be much more toxicologically and environmentally benign than most of the herbicides replaced by glyphosate.[88][relevant?]
Glyphosate can harm the bacterial ecology of soil and cause micronutrient deficiencies in plants.[89] Other studies found that while "recommended dosages of glyphosate did not affect growth rates", much higher dosages reduced respiration in nitrogen-fixing bacteria.[90][91]
The burrowing earthworm, Lumbricus terrestris, stop their activity after 3 weeks of exposure to glyphosate.[clarification needed] Reproduction of two earthworm species was reduced by 56% within three months after glyphosate. This led to increased nitrate soil concentrations by 1,592% and phosphate by 127%, for nutrient leaching into water.[92] Laboratory studies published in 1991 and 1992 indicated GBFs could harm beneficial insects[93] and earthworms.[94] The findings of negative effects of glyphosate on earthworms is cricized[85] and said to conflict with results from 1989 field studies where no effects were noted for a number of nematodes, mites, or [[springtail]s after treatment with Roundup at 2 kg/ha of active ingredient.[95] Monsanto's education sheet on soil microbes from 2011 argues that abundant tests have been done to satisfy regulators, that laboratory based tests are inferior to field tests and focuses on an "undisturbed" Rhizobium relationship.[96]
Antimetabolic activity, plants
Carbon metabolism is inhibited in glyphosate-treated plants, causing carbohydrates to collect and pool in the leaves and roots with alcoholic fermentation in the roots.[97]
Genetic damage
Glyphosate produces DNA strand breaks in the comet assay in sábalo, European eel, zebrafish, Nile tilapia.[98]
Government and organization positions on glyphosate toxicity
European Food Safety Authority position
A 2013 systematic review by the German Institute for Risk Assessment (BfR), conducted as part of the EFSA's review process, examined epidemiological studies, animal studies, and in vitro studies that it found valid, and found that "no classification and labelling for carcinogenicity is warranted" and did not recommend a carcinogen classification of either 1A or 1B.[9]: Volume 1, p139, see also 34–37 It was provided to the EFSA in January 2014 and published by the EFSA in December 2014[9][99][100]
US Environmental Protection Agency position
The EPA, which last reviewed glyphosate in 1993, considers glyphosate to be noncarcinogenic and relatively low in dermal and oral acute toxicity.[33] The EPA considered a "worst case" dietary risk model of an individual eating a lifetime of food derived entirely from glyphosate-sprayed fields with residues at their maximum levels. This model indicated that no adverse health effects would be expected under such conditions.[33] As of March 2015, the EPA was in the midst of reviewing glyphosate's toxicity.[5]
World Health Organization position
In March 2015, the International Agency for Research on Cancer published a summary of their forthcoming monograph on glyphosate, and classified glyphosate as "probably carcinogenic in humans" (category 2A) based on epidemiological studies, animal studies, and in vitro studies; it noted that there was "limited evidence" of carcinogenicity in humans for non-Hodgkin lymphoma.[5][11][12][101] The German Institute for Risk Assessment responded that the work group reviewed only a selection of what they had reviewed earlier, and argued that other studies, among them the widely-cited cohort study Agricultural Health Study, do not support the classification.[102] The IARC report did not include the German regulatory study published in December 2014, nor did it include industry-funded studies. The IARC also does not conduct risk assessment; their goal is to classify carcinogenic potential, and "a few positive findings can be enough to declare a hazard, even if there are negative studies as well."[103]
Additive toxicity
Glyphosate formulations may contain a number of adjuvants, the identity of which is considered a trade secret and not disclosed by government regulators. In the United States, the Federal Insecticide, Fungicide, and Rodenticide Act requires that all pesticides (including herbicides) be evaluated by the EPA prior to sale, including product’s chemistry, environmental fate, residue chemistry, dietary and nondietary hazards to humans, and hazards to domestic animals and nontarget organisms[104] These evaluations are performed for each active ingredient, each inert ingredient, and for the final product formulation. Additional evaluations are performed by the FDA to set permitted residue levels in food for pesticide products used on food crops.[105]
Surfactants
Surfactants are used in herbicide formulations as wetting agents, to maximize coverage and aid penetration of the herbicide(s) through plant leaves. As agricultural spray adjuvants, surfactants may be premixed in commercial formulations, such as Roundup, or they may be purchased separately and mixed on-site (tank mix).
Polyethoxylated tallow amine (POEA) is a surfactant used in the original Roundup formulation and is still commonly used today.[106] Different versions of Roundup have included different percentages of POEA. Although Monsanto product fact sheets do not disclose surfactants and their percentages, a 1997 US government report said that Roundup is 15% POEA while Roundup Pro is 14.5%.[107]
A review of the literature provided to the EPA in 1997 found that POEA was more toxic to fish than glyphosate was.[107]
Spreader 90 is a surfactant used in tank mixes.[111] Spreader 90 contains 1,2 propanediol (also known as propylene glycol), propane 1,2,3 triol (also known as glycerol), alcohol ethoxylate, and dimethylpolysiloxane.[112][113] Of these ingredients, alcohol ethoxylates are among the widely used detergents in consumer products; commercial preparations are often mixes of homologs. Due to known toxicities to aquatic species, the Canadian Environmental Protection Act, 1999 recommended Federal Water Quality Guideline values of 70 µg/l.[114]
Inert ingredient toxicity
Glyphosate-based formulations (GBFs) may contain a number of adjuvants, the identities of which are considered trade secret[115] and not disclosed by most government regulators. In the United States, the Federal Insecticide, Fungicide, and Rodenticide Act requires that all pesticides (including herbicides) be evaluated by the EPA prior to sale, including the product’s chemistry, environmental fate, residue chemistry, dietary and non-dietary hazards to humans, and hazards to domestic animals and non-target organisms.[104] These evaluations are performed for each active ingredient, each inert ingredient, and for the final product formulation. Additional evaluations are performed by the FDA to set permitted residue levels in food for pesticide products used on food crops.[105]
Surfactants are used in herbicide formulations as wetting agents and penetrants to maximize coverage and aid penetration of the herbicide(s) through plant leaves. As agricultural spray adjuvants, surfactants may be premixed in commercial formulations or they may be purchased separately and mixed on-site (tank mix).
Polyethoxylated tallow amine (POEA) is a surfactant used in the original Roundup formulation and is still commonly used today.[106] Different versions of Roundup have included different percentages of POEA. Although Monsanto product fact sheets do not disclose surfactants and their percentages, a 1997 US government report said that Roundup is 15% POEA while Roundup Pro is 14.5%.[107] A review of the literature provided to the EPA in 1997 found that POEA was more toxic to fish than glyphosate was.[107]
Spreader 90 is a surfactant used in tank mixes.[116] Spreader 90 contains 1,2 propanediol (also known as propylene glycol), propane 1,2,3 triol (also known as glycerol), alcohol ethoxylate, and dimethylpolysiloxane.[112][113] Of these ingredients, alcohol ethoxylates are among the widely used detergents in consumer products; commercial preparations are often mixes of homologs. Due to known toxicities to aquatic species, the Canadian Environmental Protection Act, 1999 recommended Federal Water Quality Guideline values of 70 µg/l.[114]
Human
A 2000 review concluded that "under present and expected conditions of new use, there is no potential for Roundup herbicide to pose a health risk to humans".[70] A 2002 review by the European Union reached the same conclusion.[71]
Data from the California Environmental Protection Agency's Pesticide Illness Surveillance Program, which also tracks other agricultural chemicals, show glyphosate-related incidents are some of the most common.[117][118] However, incident counts alone do not take into account the number of people exposed and the severity of symptoms associated with each incident.[118] For example, if hospitalization were used as a measure of the severity of incidents, then glyphosate would be considered relatively safe; over a 13-year period in California, none of the 515 reported hospitalizations was attributed to glyphosate.[118]
Dermal exposure to ready-to-use glyphosate formulations can cause irritation, and photocontact dermatitis has been occasionally reported. These effects are probably due to the preservative benzisothiazolin-3-one. Severe skin burns are very rare.[63] Inhalation is a minor route of exposure, but spray mist may cause oral or nasal discomfort, an unpleasant taste in the mouth, or tingling and irritation in the throat. Eye exposure may lead to mild conjunctivitis. Superficial corneal injury is possible if irrigation is delayed or inadequate.[63]
Deliberate ingestion of Roundup in quantities ranging from 85 to 200 ml (of 41% solution) has resulted in death within hours of ingestion, although it has also been ingested in quantities as large as 500 ml with only mild or moderate symptoms.[119] A reasonable correlation is seen between the amount of Roundup ingested and the likelihood of serious systemic sequelae or death. Ingestion of more than 85 ml of the concentrated formulation is likely to cause significant toxicity in adults. Corrosive effects – mouth, throat and epigastric pain and dysphagia – are common. Renal and hepatic impairment are also frequent, and usually reflect reduced organ perfusion. Respiratory distress, impaired consciousness, pulmonary edema, infiltration on chest X-ray, shock, arrhythmias, renal failure requiring haemodialysis, metabolic acidosis, and hyperkalaemia may occur in severe cases. Bradycardia and ventricular arrhythmias often present prior to death.
A 2012 meta-analysis of all epidemiological studies of exposure to glyphosate formulations found no correlation with any kind of cancer.[66] The 2013 systematic review by the German Institute for Risk Assessment of epidemiological studies of workers who use pesticides, exposed to glyphosate formulations found no significant risk, stating that "the available data is contradictory and far from being convincing".[9]: Volume 1, p64-66 However, a 2014 meta-analysis of the same studies found a correlation between occupational exposure to glyphosate formulations and increased risk of B cell lymphoma, the most common kind of non-Hodgkin lymphoma (NHL). Workers exposed to glyphosate were about twice as likely to get B cell lymphoma.[10]
Endocrine disruption
Administering Roundup Transorb orally to prepubescent rats once a day for 30 days reduced testosterone production and affected testicle morphology, but did not affect levels of estradiol and corticosterone.[120]
In 2007, the EPA selected glyphosate for further screening through its Endocrine Disruptor Screening Program (EDSP). Selection for this program is based on a compound's prevalence of use and does not imply particular suspicion of endocrine activity.[121] On June 29, 2015 the EPA released Weight of Evidence Conclusion of the EDSP Tier 1 screening for glyphosate, recommending that glyphosate not be considered for Tier 2 testing. The Weight of Evidence conclusion stated "...there was no convincing evidence of potential interaction with the estrogen, androgen or thyroid pathways."[122]
Genetic damage
Several studies have not found mutagenic effects,[123] so glyphosate has not been listed in the United States Environmental Protection Agency or the International Agency for Research on Cancer databases.[124] Various other studies suggest glyphosate may be mutagenic.[124] Glyphosate-based formulations produce DNA strand breaks in several major taxa of animals including numerous fish species (European eel, sábalo, guppy, bloch, neotropical fish (Corydoras paleatus) carp, goldfish), reptiles (caiman), amphibians (frogs, including tadpoles) and gastropods (snails), but not in oysters, clams and mussel glochidia. In earthworms, one glyphosate-based formulation induced DNA strand breaks while two others did not, thereby emphasising the potential importance of components other than the active ingredients in the formulations.[125]
Other animals
A 2000 review of the ecotoxicological data on Roundup shows at least 58 studies exist on the effects of Roundup on a range of organisms.[85] This review concluded, "...for terrestrial uses of Roundup minimal acute and chronic risk was predicted for potentially exposed non-target organisms".
Fish and aquatic life
Glyphosate formulations are much more toxic for amphibians and fish than glyphosate alone.[107] Glyphosate formulations may contain a number of so-called ‘inert’ ingredients or adjuvants, most of which are not publicly known as in many countries the law does not require that they be revealed.[72] A 2003 study of various formulations of glyphosate found, "[the] risk assessments based on estimated and measured concentrations of glyphosate that would result from its use for the control of undesirable plants in wetlands and over-water situations showed that the risk to aquatic organisms is negligible or small at application rates less than 4 kg/ha and only slightly greater at application rates of 8 kg/ha.".[126] Serious sub-lethal effects on aquatic animals have been noted for formulations of glyphosate.[73]
The LC50 of formulations for Catla catla is 4.6 ppm,[127] 4.2 ppm for rainbow trout (Oncorhynchus mykiss) and 1.3 ppm for bluegill (Lepomis macrochirus)[128] The 96 h-LC50 toxicity of Roundup for the neotropical fish Prochilodus lineatus is 13.69 mg/L, indicating this fish is more sensitive to Roundup than rainbow trout and Atlantic salmon (Salmo salar).[129] Short-term exposure of Prochilodus lineatus to sub-lethal concentrations of Roundup results in biochemical, physiological and histological alterations. Histopathological changes are observed in the gills, livers and brains in other fishes at these concentrations.[130]
A 2013 meta-analysis reviewed the available data related to potential impacts of glyphosate-based herbicides on amphibians. According to the authors, the use of glyphosate-based pesticides cannot be considered the major cause of amphibian decline, the bulk of which occurred prior to the widespread use of glyphosate or in pristine tropical areas with minimal glyphosate exposure. The authors recommended further study of species- and development-stage chronic toxicity, of environmental glyphosate levels, and ongoing analysis of data relevant to determining what if any role glyphosate might be playing in worldwide amphibian decline, and suggest including amphibians in standardized test batteries.[131]
Glyphosate-based formulations can cause oxidative stress in bullfrog tadpoles and Pacific oysters.[132]
In reproductive toxicity studies performed in rats and rabbits, no adverse maternal or offspring effects were seen at doses below 175–293 mg/kg of body weight per day.[7]
Monsanto and other companies produce glyphosate products with alternative surfactants specifically formulated for aquatic use, for example the Monsanto products "Biactive" and "AquaMaster".[133][134] In 2001, the Monsanto product Vision® was studied in a forest wetlands site in Canada. Substantial mortality occurred only at concentrations exceeding the expected environmental concentrations as calculated by Canadian regulatory authorities. While it was found that site factors such as pH and suspended sediments substantially affected the toxicity in the amphibian larvae tested, overall, "results suggest that the silvicultural use of Vision herbicide in accordance with the product label and standard Canadian environmental regulations should have negligible adverse effects on sensitive larval life stages of native amphibians."[135]
A 2003 study of various formulations of glyphosate found, "[the] risk assessments based on estimated and measured concentrations of glyphosate that would result from its use for the control of undesirable plants in wetlands and over-water situations showed that the risk to aquatic organisms is negligible or small at application rates less than 4 kg/ha and only slightly greater at application rates of 8 kg/ha.".[126] A 2013 meta-analysis also reviewed the available data related to potential impacts of glyphosate-based herbicides on amphibians. According to the authors, the use of glyphosate-based pesticides cannot be considered the major cause of amphibian decline, the bulk of which occurred prior to the widespread use of glyphosate or in pristine tropical areas with minimal glyphosate exposure. The authors recommended further study of species- and development-stage chronic toxicity, of environmental glyphosate levels, and ongoing analysis of data relevant to determining what if any role glyphosate might be playing in worldwide amphibian decline, and suggest including amphibians in standardized test batteries.[131]
Glyphosate formulations are much more toxic for amphibians and fish than glyphosate alone.[107] Glyphosate formulations may contain a number of so-called ‘inert’ ingredients or adjuvants, most of which are not publicly known as in many countries the law does not require that they be revealed.[72]
A study published in 2010 proposed commercial glyphosate can cause neural defects and craniofacial malformations in African clawed frogs (Xenopus laevis). The experiments used frog embryos that were incubated with 1:5000 dilutions of a commercial glyphosate solution. The frog embryos suffered diminution of body size, alterations of brain morphology, reduction of the eyes, alterations of the branchial arches and otic placodes, alterations of the neural plate, and other abnormalities of the nervous system. The authors suggested glyphosate itself was responsible for the observed results because injection of pure glyphosate produced similar results in a chicken model.[136]
Effect on plant health
A correlation was found between an increase in the infection rate of wheat by Fusarium head blight and the application of glyphosate, but "because of the nature of this study, we could not determine if the association between previous GF (glyphosate formulation) use and FHB development was a cause-effect relationship".[137] Other studies have found causal relationships between glyphosate and decreased disease resistance.[138] Exposure to glyphosate has been shown to change the species composition of endophytic bacteria in plant hosts, which is highly variable.[139]
Effects of use
Resistance
Resistance evolves after a weed population has been subjected to intense selection pressure in the form of repeated use of a single herbicide.[140][141] Weeds resistant to the herbicide have been called 'superweeds'.[142] The first documented cases of weed resistance to glyphosate were found in Australia in 1996, involving rigid ryegrass (Lolium rigidum) near Orange, New South Wales.[143][144][145] In 2006, farmers associations were reporting 107 biotypes of weeds within 63 weed species with herbicide resistance.[146] In 2009, Canada identified its first resistant weed, giant ragweed, and at that time 15 weed species had been confirmed as resistant to glyphosate.[140][147] As of 2010, in the United States 7 to 10 million acres (28,000 to 40,000 km2) of soil were afflicted by superweeds, or about 5% of the 170 million acres planted with corn, soybeans, and cotton, the crops most affected, in 22 states.[148] In 2012, Charles Benbrook reported that the Weed Science Society of America listed 22 super weeds in the U.S., with over 5.7 million has (14 million ac) infested by GR weeds and that Dow AgroSciences had carried out a survey and reported a figure of around 40 million ha (100 million ac).[149] As of 2014, the International Survey of Herbicide Resistant Weeds database listed 211 weeds that were resistant to glyphosate.[150]
In response to resistant weeds, farmers are hand-weeding, using tractors to turn over soil between crops, and using other herbicides in addition to glyphosate.
Monsanto scientists have some resistant weeds that have as many as 160 extra copies of a gene called EPSPS, the enzyme glyphosate disrupts.[151]
Palmer amaranth

In 2004, a glyphosate-resistant variation of Amaranthus palmeri, commonly known as Palmer amaranth, was found in Georgia and confirmed by a 2005 study.[152] In 2005, resistance was also found in North Carolina.[153] Widespread use of Roundup Ready crops led to an unprecedented selection pressure, and glyphosate resistance followed.[153] The weed variation is now widespread in the southeastern United States.[154] Cases have also been reported in Texas[154] and Virginia.[155]
Conyza

Conyza bonariensis (also known as hairy fleabane and buva) and Conyza canadensis (known as horseweed or marestail), are other weed species that had lately developed glyphosate resistance.[156][157][158] A 2008 study on the current situation of glyphosate resistance in South America concluded "resistance evolution followed intense glyphosate use" and the use of glyphosate-resistant soybean crops is a factor encouraging increases in glyphosate use.[159] In the 2015 growing season, glyphosate-resistant marestail proved to be especially problematic to control in Nebraska production fields.[160]
Ryegrass

Glyphosate-resistant ryegrass (Lolium) has occurred in most of the Australian agricultural areas and other areas of the world. All cases of evolution of resistance to glyphosate in Australia were characterized by intensive use of the herbicide while no other effective weed control practices were used. Studies indicate the resistant ryegrass does not compete well against nonresistant plants and their numbers decrease when not grown under conditions of glyphosate application.[161]
Johnson grass
Glyphosate-resistant Johnson grass (Sorghum halepense) is found in glyphosate-resistant soybean cultivation in northern Argentina.[162]
Monarch Butterfly
Use of glyphosate to clear milkweed along roads and fields has led to a decline in monarch butterfly populations in the Midwest. The herbicide usage caused an estimated 58% decline in milkweeds, which resulted in 81% decline in monarchs.[163][164] The Natural Resources Defense Council (NRDC) filed a suit in 2015 against the EPA, in which it is argued that the agency ignored warnings about the dangers of glyphosate usage for monarchs.[165]
Legal status
Glyphosate was first approved for use in the 1970s, and as of 2010 was labelled for use in 130 countries.[13]: 2
In September 2013 the legislative assembly of El Salvador approved legislation to ban 53 agrochemicals, including glyphosate; the ban on glyphosate was set to begin in 2015.[166][167][168]
In April 2014 the legislature of the Netherlands passed legislation prohibiting sale of glyphosate to individuals for use at home; commercial sales were not affected.[169]
In May 2015 the president of Sri Lanka banned the use and import of glyphosate, effective immediately.[170][171]
In May 2015, Bermuda blocked importation on all new orders of glyphosate-based herbicides for a temporary suspension awaiting outcomes of research.[172]
In May 2015, Colombia announced that it would stop using glyphosate by October 2015 in the destruction of illegal plantations of coca, the raw ingredient for cocaine. Farmers have complained that the aerial fumigation has destroyed entire fields of coffee and other legal produce.[173]
In June 2015, the French Ecology Minister asked nurseries and garden centers to sell glyphosate only from locked cabinets. This was only a request and all sales of glyphosate remained legal in France.[174]
Legal cases
Advertising controversy
The New York Times reported that in 1996, "Dennis C. Vacco, the Attorney General of New York, ordered the company Monsanto to pull ads that said Roundup was "safer than table salt" and "practically nontoxic" to mammals, birds and fish. The company withdrew the spots, but also said that the phrase in question was permissible under E.P.A. guidelines."[175]
In 2001, French environmental and consumer rights campaigners brought a case against Monsanto for misleading the public about the environmental impact of its herbicide Roundup, on the basis that glyphosate, Roundup's main component, is classed as "dangerous for the environment" and "toxic for aquatic organisms" by the European Union. Monsanto's advertising for Roundup had presented it as biodegradable and as leaving the soil clean after use. In 2007, Monsanto was convicted of false advertising and was fined 15,000 euros. Monsanto's French distributor Scotts France was also fined 15,000 euros. Both defendants were ordered to pay damages of 5,000 euros to the Brittany Water and Rivers Association and 3,000 euros to the CLCV (Consommation Logement Cadre de vie), one of the two main general consumer associations in France.[176] Monsanto appealed and the court upheld the verdict; Monsanto appealed again to the French Supreme Court, and in 2009 it also upheld the verdict.[177]
Scientific fraud
On two occasions, the United States EPA has caught scientists deliberately falsifying test results at research laboratories hired by Monsanto to study glyphosate.[178] The first incident involved Industrial Biotest Laboratories (IBT). The United States Justice Department closed the laboratory in 1978, and its leadership was found guilty in 1983 of charges of falsifying statements, falsifying scientific data submitted to the government, and mail fraud.[179]
In 1991, Don Craven, the owner of Craven Laboratories and three employees were indicted on 20 felony counts. Craven, along with fourteen employees were found guilty of similar crimes.[180]
Monsanto has stated the Craven Labs investigation was started by the EPA after a pesticide industry task force discovered irregularities, that the studies have been repeated, and that Roundup's EPA certification does not now use any studies from Craven Labs or IBT.[178]
Trade dumping allegations
United States companies have cited trade issues with glyphosate being dumped into the western world market areas by Chinese companies and a formal dispute was filed in 2010.[181][182]
Genetically modified crops
Some micro-organisms have a version of 5-enolpyruvoyl-shikimate-3-phosphate synthetase (EPSPS) resistant to glyphosate inhibition. A version of the enzyme that both was resistant to glyphosate and that was still efficient enough to drive adequate plant growth was identified by Monsanto scientists after much trial and error in an Agrobacterium strain called CP4, which was found surviving in a waste-fed column at a glyphosate production facility.[183][184][185]: 56 This CP4 EPSPS gene was cloned and transfected into soybeans. In 1996, genetically modified soybeans were made commercially available.[186] Current glyphosate-resistant crops include soy, maize (corn), canola, alfalfa, and cotton, with wheat still under development.
Genetically modified crops have become the norm in the United States. In 2015, 89% of corn, 94% of soybeans, and 89% of cotton produced in the US were genetically modified to be herbicide-tolerant.[187]
See also
- 2,4-Dichlorophenoxyacetic acid
- Ammonium sulfamate
- Atrazine
- Environmental impact of pesticides
- Health effects of pesticides
- Integrated pest management
- Séralini affair
External links
- Glyphosate in the Pesticide Properties DataBase (PPDB)
- Glyphosate trimesium in the Pesticide Properties DataBase (PPDB)
- Glyphosate, isopropylamine salt in the Pesticide Properties DataBase (PPDB)
- Glyphosate, potassium salt in the Pesticide Properties DataBase (PPDB)
References
- ^ a b Glyphosate, Environmental Health Criteria monograph No. 159, Geneva: World Health Organization, 1994, ISBN 92-4-157159-4
- ^ Index no. 607-315-00-8 of Annex VI, Part 3, to Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJEU L353, 31.12.2008, pp 1–1355 at pp 570, 1100..
- ^ US patent 3799758, Franz JE, "N-phosphonomethyl-glycine phytotoxicant compositions", issued 1974-03-26, assigned to Monsanto Company
- ^ United States EPA 2007 Pesticide Market Estimates Agriculture, Home and Garden
- ^ a b c d Daniel Cressey, Widely used herbicide linked to cancer, Nature, 24 March 2015.
- ^ Sprankle, Paul, W. F. Meggitt, and Donald Penner. "Rapid inactivation of glyphosate in the soil." Weed Science (1975): 224-228.
- ^ a b c d e f g h i "Glyphosate technical fact sheet (revised June 2015)". National Pesticide Information Center. 2010. Retrieved September 1, 2015.
- ^ a b c d "The agronomic benefits of glyphosate in Europe" (PDF). Monsanto Europe SA. February 2010. Retrieved 2013-06-02.
- ^ a b c d Renewal Assessment Report: Glyphosate, Volume 1, Report and Proposed Decision", German Institute for Risk Assessment, 18 December 2013, p. 65. Download available from EFSA Provision of documents (registration required): Volume 1, pp. 64–66 Cite error: The named reference "BFR2014" was defined multiple times with different content (see the help page).
- ^ a b c Schinasi, L (April 23, 2014). "Non-Hodgkin lymphoma and occupational exposure to agricultural pesticide chemical groups and active ingredients: a systematic review and meta-analysis". International Journal of Environmental Research and Public Health. 11 (4): 4449. doi:10.3390/ijerph110404449. PMID 24762670. Retrieved 31 August 2015.
{{cite journal}}: CS1 maint: unflagged free DOI (link) Cite error: The named reference "Schinasi" was defined multiple times with different content (see the help page). - ^ a b c Guyton KZ, Loomis D, Grosse Y, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Scoccianti C, Mattock H, Straif K (May 2015). "Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate". The Lancet. Oncology. 16 (5): 490–1. doi:10.1016/S1470-2045(15)70134-8. PMID 25801782.
- ^ a b "Press release: IARC Monographs Volume 112: evaluation of five organophosphate insecticides and herbicides" (PDF). International Agency for Research on Cancer, World Health Organization. March 20, 2015.
- ^ a b c Dill GM et al. Glyphosate: Discovery, Development, Applications, and Properties. Chapter 1 in Glyphosate Resistance in Crops and Weeds: History, Development, and Management, Vijay K. Nandula (Editor). Wiley, September 2010 ISBN 978-0-470-41031-8
- ^ United States Patent 3,160,632 (1964) Stauffer Chemical
- ^ Alibhai MF, Stallings WC (Mar 2001). "Closing down on glyphosate inhibition--with a new structure for drug discovery". Proceedings of the National Academy of Sciences of the United States of America. 98 (6): 2944–6. Bibcode:2001PNAS...98.2944A. doi:10.1073/pnas.061025898. JSTOR 3055165. PMC 33334. PMID 11248008.
- ^ Stephen O Duke and Stephen B. Powles (2008) Glyphosate: a once-in-a-century herbicide: Mini-review. Pest Management Science Pest Manag Sci 64:319–325
- ^ Monsanto's John E. Franz Wins 1990 Perkin Medal Chem. Eng. News, 1990, 68 (11), pp 29–30 doi:10.1021/cen-v068n011.p029
- ^ Pesticide Action Network UK.Glyphosate fact sheet Pesticides News No.33, September 1996, p28-29 PAN-UK says it is "a welcome move away from chemicals which are highly toxic to humans and other non target organisms, and from chemicals which cause direct and lasting damage to the environment" and of course cautions against overuse.
- ^ Dr. Kathleen A. Marrs What is Biology Good For? Controlling Weeds: RoundUp
- ^ "The National Medal of Technology and Innovation Recipients - 1987". The United States Patent and Trademark Office. Retrieved 2012-11-29.
- ^ Stong C (May 1990). "People: Monsanto Scientist John E. Franz Wins 1990 Perkin Medal For Applied Chemistry". The Scientist. 4 (10): 28.
- ^ "Meet the 2007 National Inventors Hall of Fame Inductees". National Inventors Hall of Fame. 2007. Archived from the original on October 5, 2013.
- ^ Schuette J. "Environmental Fate of Glyphosate" (PDF). Department of Pesticide Regulation, State of California.
- ^ Steinrücken HC, Amrhein N (Jun 1980). "The herbicide glyphosate is a potent inhibitor of 5-enolpyruvyl-shikimic acid-3-phosphate synthase". Biochemical and Biophysical Research Communications. 94 (4): 1207–12. doi:10.1016/0006-291X(80)90547-1. PMID 7396959.
- ^ Purdue University, Department of Horticulture and Landscape Architecture, Metabolic Plant Physiology Lecture notes, Aromatic amino acid biosynthesis, The shikimate pathway – synthesis of chorismate.
- ^ Schönbrunn E, Eschenburg S, Shuttleworth WA, Schloss JV, Amrhein N, Evans JN, Kabsch W (Feb 2001). "Interaction of the herbicide glyphosate with its target enzyme 5-enolpyruvylshikimate 3-phosphate synthase in atomic detail". Proceedings of the National Academy of Sciences of the United States of America. 98 (4): 1376–80. Bibcode:2001PNAS...98.1376S. doi:10.1073/pnas.98.4.1376. PMC 29264. PMID 11171958.
- ^ Glyphosate bound to proteins in the Protein Data Bank
- ^ Schulz, A., A. Krüper, and N. Amrhein. "Differential sensitivity of bacterial 5-enolpyruvylshikimate-3-phosphate synthases to the herbicide glyphosate." FEMS Microbiology Letters 28.3 (1985): 297-301.
- ^ Pollegioni L et al. Molecular basis of glyphosate resistance-different approaches through protein engineering. FEBS J. 2011 Aug;278(16):2753-66. PMID 21668647 PMC 3145815
- ^ Funke T, Han H, Healy-Fried ML, Fischer M, Schönbrunn E (Aug 2006). "Molecular basis for the herbicide resistance of Roundup Ready crops". Proceedings of the National Academy of Sciences of the United States of America. 103 (35): 13010–5. Bibcode:2006PNAS..10313010. doi:10.1073/pnas.0603638103. JSTOR 30050705. PMC 1559744. PMID 16916934.
{{cite journal}}: Check|bibcode=length (help) - ^ Maeda H1, Dudareva N. The shikimate pathway and aromatic amino Acid biosynthesis in plants. Annu Rev Plant Biol. 2012;63:73-105. doi: 10.1146/annurev-arplant-042811-105439. PMID 22554242 quote: "The AAA pathways consist of the shikimate pathway (the prechorismate pathway) and individual postchorismate pathways leading to Trp, Phe, and Tyr.... These pathways are found in bacteria, fungi, plants, and some protists but are absent in animals. Therefore, AAAs and some of their derivatives (vitamins) are essential nutrients in the human diet, although in animals Tyr can be synthesized from Phe by Phe hydroxylase....The absence of the AAA pathways in animals also makes these pathways attractive targets for antimicrobial agents and herbicides."
- ^ Cerdeira AL, Duke SO (2006). "The current status and environmental impacts of glyphosate-resistant crops: a review". Journal of Environmental Quality. 35 (5): 1633–58. doi:10.2134/jeq2005.0378. PMID 16899736.
- ^ a b c d e "Registration Decision Fact Sheet for Glyphosate (EPA-738-F-93-011)" (PDF). R.E.D. FACTS. United States Environmental Protection Agency. 1993.
- ^ Borggaard OK, Gimsing AL (Apr 2008). "Fate of glyphosate in soil and the possibility of leaching to ground and surface waters: a review". Pest Management Science. 64 (4): 441–56. doi:10.1002/ps.1512. PMID 18161065.
- ^ a b Andréa MM, Peres TB, Luchini LC, Bazarin S, Papini S, Matallo MB, Savoy VLT (2003). "Influence of repeated applications of glyphosate on its persistence and soil bioactivity". Pesquisa Agropecuária Brasileira. 38 (11): 1329–1335. doi:10.1590/S0100-204X2003001100012.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Torstensson NT, Lundgren LN, Stenström J (Oct 1989). "Influence of climatic and edaphic factors on persistence of glyphosate and 2,4-D in forest soils". Ecotoxicology and Environmental Safety. 18 (2): 230–9. doi:10.1016/0147-6513(89)90084-5. PMID 2806176.
- ^ Albers CN, Banta GT, Hansen PE, Jacobsen OS (Oct 2009). "The influence of organic matter on sorption and fate of glyphosate in soil--comparing different soils and humic substances". Environmental Pollution. 157 (10): 2865–70. doi:10.1016/j.envpol.2009.04.004. PMID 19447533.
- ^ Ole K. Borggaard OK (2011). "Does phosphate affect soil sorption and degradation of glyphosate? - A review". Trends in Soil Science and Plant Nutrition. 2 (1): 17–27.
- ^ Doublet J, Mamy L, Barriuso E (Oct 2009). "Delayed degradation in soil of foliar herbicides glyphosate and sulcotrione previously absorbed by plants: consequences on herbicide fate and risk assessment". Chemosphere. 77 (4): 582–9. doi:10.1016/j.chemosphere.2009.06.044. PMID 19625069.
- ^ Thomas M Amrein (2012-12-21). "Analysis of pesticides in food" (PDF). ETH Zurich. p. 15. Retrieved 2013-06-02.
- ^ Stevan Z. Knezevic, University of Nebraska Extension Integrated Weed Management Specialist, last revised Revised February 2010 Use of Herbicide-Tolerant Crops as Part of an Integrated Weed Management Program
- ^ Luijendijk CD et al Measures to reduce glyphosate runoff from hard surfaces Plant Research International B.V., Wageningen May 2005
- ^ Botta F, Lavison G, Couturier G, Alliot F, Moreau-Guigon E, Fauchon N, Guery B, Chevreuil M, Blanchoud H (Sep 2009). "Transfer of glyphosate and its degradate AMPA to surface waters through urban sewerage systems". Chemosphere. 77 (1): 133–9. doi:10.1016/j.chemosphere.2009.05.008. PMID 19482331.
- ^ BBC. May 10, 2015. Colombia to ban coca spraying herbicide glyphosate
- ^ Louisiana State University Agricultural Extension Office. Last Updated: 3 September 2014 Sugarcane Ripener Recommendations - Glyphosate Page Accessed 3 September 2014
- ^ Farm Chemicals International Glyphosate entry in Crop Protection Database
- ^ a b Alberta Agriculture and Rural Development. April 26, 2006. Quick Guide to Glyphosate Products - Frequently Asked Questions
- ^ Hartzler B. "ISU Weed Science Online - Glyphosate - A Review". Iowa State University Extension.
- ^ a b c Tu M, Hurd C, Robison R, Randall JM (2001-11-01). "Glyphosate" (PDF). Weed Control Methods Handbook. The Nature Conservancy.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ National Pesticide Information Center. Last updated September 2010 Glyphosate General Fact Sheet
- ^ a b c Reuters. Apr 30, 2014. Press Release: Research and Markets: Global Glyphosate Market for Genetically Modified and Conventional Crops 2013 - 2019
- ^ China Research & Intelligence, June 5, 2013. Research Report on Global and China Glyphosate Industry, 2013-2017
- ^ a b VanGessel M. "Glyphosate Formulations". Control Methods Handbook, Chapter 8, Adjuvants: Weekly Crop Update. University of Delaware Cooperative Extension.
- ^ Tu M, Randall JM (2003-06-01). "Glyphosate" (PDF). Weed Control Methods Handbook. The Nature Conservancy.
- ^ Curran WS, McGlamery MD, Liebl RA, Lingenfelter DD (1999). "Adjuvants for Enhancing Herbicide Performance". Penn State Extension.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ Sprague C, Hager A (2000-05-12). "Principles of Postemergence Herbicides". University of Illinois Extension Service. Retrieved 2012-11-29.
- ^ Young B. "Adjuvant Products by Manufacturer, Compendium of Herbicide Adjuvants". Southern Illinois University.
- ^ e-phy: Le catalogue des produits phytopharmaceutiques et de leurs usages des matières fertilisantes et des supports de culture homologués en France
- ^ "The debate over whether Monsanto is a corporate sinner or saint". The Economist. 19 November 2009. Retrieved 20 November 2009.
- ^ Cavallaro M (2009-06-26). "The Seeds Of A Monsanto Short Play". Forbes. Retrieved 2009-07-11.
- ^ Kimmel, Gary (2013). "Evaluation of developmental toxicity studies of glyphosate with attention to cardiovascular development". Critical Reviews in Toxicology. 43 (2): 79–95. doi:10.3109/10408444.2012.749834.
- ^ Mesnage, R., Defarge, N., Spiroux de Vendômois, J. and Séralini, G.E. (2015). "Potential toxic effects of glyphosate and its commercial formulations below regulatory limits". Food and Chemical Toxicology. 84: 133–153. doi:10.1016/j.fct.2015.08.012. PMID 26282372.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Cite error: The named reference
Bradberry_2004was invoked but never defined (see the help page). - ^ Sribanditmongkol P, Jutavijittum P, Pongraveevongsa P, Wunnapuk K, Durongkadech P (Sep 2012). "Pathological and toxicological findings in glyphosate-surfactant herbicide fatality: a case report". The American Journal of Forensic Medicine and Pathology. 33 (3): 234–7. doi:10.1097/PAF.0b013e31824b936c. PMID 22835958.
- ^ Mink PJ, Mandel JS, Lundin JI, Sceurman BK (Nov 2011). "Epidemiologic studies of glyphosate and non-cancer health outcomes: a review". Regulatory Toxicology and Pharmacology. 61 (2): 172–84. doi:10.1016/j.yrtph.2011.07.006. PMID 21798302.
- ^ a b Mink PJ, Mandel JS, Sceurman BK, Lundin JI (Aug 2012). "Epidemiologic studies of glyphosate and cancer: a review". Regulatory Toxicology and Pharmacology. 63 (3): 440–52. doi:10.1016/j.yrtph.2012.05.012. PMID 22683395.
- ^ Williams AL, Watson RE, DeSesso JM (2012). "Developmental and reproductive outcomes in humans and animals after glyphosate exposure: a critical analysis". Journal of Toxicology and Environmental Health. Part B, Critical Reviews. 15 (1): 39–96. doi:10.1080/10937404.2012.632361. PMID 22202229.
- ^ Kimmel GL, Kimmel CA, Williams AL, DeSesso JM (Feb 2013). "Evaluation of developmental toxicity studies of glyphosate with attention to cardiovascular development". Critical Reviews in Toxicology. 43 (2): 79–95. doi:10.3109/10408444.2012.749834. PMC 3581053. PMID 23286529.
- ^ Kier LD, Kirkland DJ (Apr 2013). "Review of genotoxicity studies of glyphosate and glyphosate-based formulations". Critical Reviews in Toxicology. 43 (4): 283–315. doi:10.3109/10408444.2013.770820. PMID 23480780.
- ^ a b Williams GM, Kroes R, Munro IC (Apr 2000). "Safety evaluation and risk assessment of the herbicide Roundup and its active ingredient, glyphosate, for humans". Regulatory Toxicology and Pharmacology. 31 (2 Pt 1): 117–65. doi:10.1006/rtph.1999.1371. PMID 10854122.
- ^ a b "Review report for the active substance glyphosate" (PDF). Commission working document. European Commission, Health and Protection Directorate-General: Directorate E – Food Safety: plant health, animal health and welfare, international questions: E1 - Plant Health. 2002-01-21.
- ^ a b c Pesticide Action Network Asia & the Pacific (PANAP) Glyphosate 2009
- ^ a b Sparling, D.W., Matson, C., Bickham, J. and Doelling‐Brown, P. (2006). "Toxicity of glyphosate as Glypro® and LI700 to red‐eared slider (Trachemys scripta elegans) embryos and early hatchlings". Environmental Toxicology and Chemistry. 25 (10): 2768–2774.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "IARC monograph on glyphosate" (PDF). IARC. Retrieved September 27, 2015.
- ^ a b Cite error: The named reference
FreshRev2014was invoked but never defined (see the help page). - ^ a b Marin-Morales, M. A., de Campos Ventura-Camargo, B. and Hoshina, M.M. (2013). "Chapter 16 - Toxicity of Herbicides: Impact on Aquatic and Soil Biota and Human Health". Herbicides–Current Research and Case Studies in Use (PDF). pp. 399–443.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Abraham William (Wildwood. Glyphosate formulations and their use for inhibition of 5-enolpyrovylshikimate-3-phosphate synthase, US Patent 7, 771, 736 B2; 2010
- ^ Roberts F, Roberts C W, Johnson J J, Kyle D E, Krell T, Coggins J R, Coombs G H, Milhous W K, Tzipori S, Ferguson D J P, et al. Evidence for the shikimate pathway in apicomplexan parasites. Nature (London) 1998;393:801–805.PMID 9655396
- ^ Fischer RS, Berry A, Gaines CG, Jensen RA.Comparative action of glyphosate as a trigger of energy drain in eubacteria.J Bacteriol. 1986 Dec;168(3):1147-54.PMID 3096971
- ^ Zablotowicz RM1, Reddy KN.Impact of glyphosate on the Bradyrhizobium japonicum symbiosis with glyphosate-resistant transgenic soybean: a minireview. J Environ Qual. 2004 May-Jun;33(3): 825-31. PMID 15224916
- ^ Nosanchuk JD1, Ovalle R, Casadevall A. Glyphosate inhibits melanization of Cryptococcus neoformans and prolongs survival of mice after systemic infection.J Infect Dis. 2001 Apr 1;183(7):1093-9.
- ^ Schrödl W, Krüger S, Konstantinova-Müller T, Shehata AA, Rulff R, Krüger M. Possible effects of glyphosate on Mucorales abundance in the rumen of dairy cows in Germany. Curr Microbiol. 2014 Dec;69(6):817-23. doi: 10.1007/s00284-014-0656-y.
- ^ Krüger M, Shehata AA, Schrödl W, Rodloff A. Glyphosate suppresses the antagonistic effect of Enterococcus spp. on Clostridium botulinum.Anaerobe. 2013 Apr;20:74-8. doi: 10.1016/j.anaerobe.2013.01.005.
- ^ Carranza CS, Barberis CL, Chiacchiera SM, Magnoli CE.Influence of the pesticides glyphosate, chlorpyrifos and atrazine on growth parameters of nonochratoxigenic Aspergillus section Nigri strains isolated from agricultural soils. J Environ Sci Health B. 2014;49(10):747-55. doi: 10.1080/03601234.2014.929860.PMID 25065826
- ^ a b c Giesy JP, Dobson S, Solomon KR (2000). "Ecotoxicological Risk Assessment for Roundup® Herbicide". Reviews of Environmental Contamination and Toxicology. Reviews of Environmental Contamination and Toxicology. 167: 35–120. doi:10.1007/978-1-4612-1156-3_2. ISBN 978-0-387-95102-7.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ STEPHANIE STROM (19 September 2013). "Misgivings About How a Weed Killer Affects the Soil". New York Times. Retrieved 4 October 2015.
Prying corn stalks from the soil with a shovel was difficult, and when the plants finally came up, their roots were trapped in a chunk of dirt. Once freed, the roots spread out flat like a fan and were studded with only a few nodules, which are critical to the exchange of nutrients.
- ^ Balthazor TM, Hallas LE (Feb 1986). "Glyphosate-degrading microorganisms from industrial activated sludge". Applied and Environmental Microbiology. 51 (2): 432–4. PMC 238888. PMID 16346999.
- ^ Cerdeira AL, Duke SO (January 2010). "Effects of glyphosate-resistant crop cultivation on soil and water quality". GM Crops. 1 (1): 16–24. doi:10.4161/gmcr.1.1.9404. PMID 21912208.
- ^ Dick R, Lorenz N, Wojno M, Lane M (2010). Microbial dynamics in soils under long-term glyphosate tolerant cropping systems (PDF). 19th World Congress of Soil Science.
{{cite conference}}: CS1 maint: multiple names: authors list (link) - ^ Santos A, Flores M (1995). "Effects of glyphosate on nitrogen fixation of free-living heterotrophic bacteria". Letters in Applied Microbiology. 20 (6): 349–52. doi:10.1111/j.1472-765X.1995.tb01318.x.
- ^ Yamada, T; Kremer, RJ; de Camargo e Castro, PR; Wood, BW (2009). "Glyphosate interactions with physiology, nutrition, and diseases of plants: Threat to agricultural sustainability?". European Journal of Agronomy. 31 (3). Elsevier: 111–113. doi:10.1016/j.eja.2009.07.004.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Gaupp-Berghausen M1, Hofer M, Rewald B, Zaller JG Glyphosate-based herbicides reduce the activity and reproduction of earthworms and lead to increased soil nutrient concentrations. Sci Rep. 2015 Aug 5;5:12886. doi:10.1038/srep12886. PMID 26243044
- ^ Hassan SA, Bigler F, Bogenschütz H, Boller E, Brun J, Calis JNM, Chiverton P, Coremans-Pelseneer J, Duso C (1991). "Results of the fifth joint pesticide testing programme carried out by the IOBC/WPRS-Working Group 'Pesticides and beneficial organisms'". Entomophaga. 36: 55–67. doi:10.1007/BF02374636.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Springett JA, Gray RAJ (1992). "Effect of repeated low doses of biocides on the earthworm Aporrectodea caliginosa in laboratory culture". Soil Biology and Biochemistry. 24 (12): 1739–1744. doi:10.1016/0038-0717(92)90180-6.
- ^ Preston CM, Trofymow JA (1989). "Effects of glyphosate (Roundup) on biological activity of forest soils". Proceedings of the Carnation Creek Workshop: 122–40. ISBN 0-7726-0917-9.
- ^ "Part III: Glyphosate and soil microbes" (PDF). Monsanto. 30 September 2011. p. 2. Retrieved 4 October 2015.
- ^ Orcaray L, Zulet A, Zabalza A, Royuela M. Impairment of carbon metabolism induced by the herbicide glyphosate.J Plant Physiol. 2012 Jan 1;169(1):27-33. doi: 10.1016/j.jplph.2011.08.009. PMID 21944839
- ^ "IARC monograph on glyphosate" (PDF). IARC. Retrieved September 27, 2015.
- ^ "Glyphosate RAR 01 Volume 1 2013-12-18 San". Renewal Assessment Report. Hungry4Pesticides. 18 December 2013. Retrieved 27 March 2015.
- ^ Bundesinstitut für Risikobewertung. Updated 15 January 2014 Frequently asked questions on the health assessment of glyphosate
- ^ Michael Specter for the New Yorker. April 10, 2015 Roundup and Risk Assessment. Quote:"‘Probable’ means that there was enough evidence to say it is more than possible, but not enough evidence to say it is a carcinogen," Aaron Blair, a lead researcher on the I.A.R.C.’s study, said. Blair, a scientist emeritus at the National Cancer Institute, has studied the effects of pesticides for years. "It means you ought to be a little concerned about" glyphosate, he said."
- ^ "Löst glyphosat Krebs aus? (announcement 007/2015)" (PDF) (in German). German Institute for Risk Assessment. 23 March 2015.
- ^ Andrew Pollack (27 March 2015). "Weed Killer, Long Cleared, Is Doubted". New York Times.
- ^ a b "Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) | Agriculture | US EPA".
- ^ a b "Pesticide Registration Manual | Pesticide Registration | US EPA".
- ^ a b "Measuring POEA, a Surfactant Mixture in Herbicide Formulations". U.S. Geological Survey.
- ^ a b c d e f Gary L. Diamond and Patrick R. Durkin February 6, 1997, under contract from the United States Department of Agriculture. Effects of Surfactants on the Toxicitiy of Glyphosate, with Specific Reference to RODEO
- ^ a b Loveland Products. X-77 Spreader Label
- ^ a b Loveland Products. Material Safety Data Sheet - X-77 Spreader
- ^ a b Loveland Products. alt X-77 Spreader Label
- ^ Note: Prior versions were known as "X-77 Spreader".[49] This product itself had various versions: one included alkylphenol ethoxylate, alcohol ethoxylate, tall oil fatty acid, 2,2' dihydroxydiethyl ethyl, and dimethylpolysiloxane.[108][109] Another version included alkylarylpolyoxyethylene, alkylpolyoxyethylene, gatty acids, glycols and dimethylpolysiloxane.[110]
- ^ a b Loveland Products. Spreader 90 Label
- ^ a b Loveland Products. Spreader 90 Material Safety Data Sheet
- ^ a b Staff, Environment Canada. February 2013 Federal Environmental Quality Guidelines: Alcohol Ethoxylates
- ^ Hoy, J., Swanson, N. and Seneff, S. (2015). "The high cost of pesticides: Human and animal disease". Poultry, Fish & Wildlife Sciences. 3 (1).
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Note: Prior versions were known as "X-77 Spreader".[49] This product itself had various versions: one included alkylphenol ethoxylate, alcohol ethoxylate, tall oil fatty acid, 2,2' dihydroxydiethyl ethyl, and dimethylpolysiloxane.[108][109] Another version included alkylarylpolyoxyethylene, alkylpolyoxyethylene, gatty acids, glycols and dimethylpolysiloxane.[110]
- ^ Goldstein DA, Acquavella JF, Mannion RM, Farmer DR (2002). "An analysis of glyphosate data from the California Environmental Protection Agency Pesticide Illness Surveillance Program". Journal of Toxicology. Clinical Toxicology. 40 (7): 885–92. doi:10.1081/CLT-120016960. PMID 12507058.
- ^ a b c "Pesticide Illness Surveillance Program". California Pesticide Illness Serveillance Program Report HS-1733. California EPA. 2010.
- ^ Talbot AR, Shiaw MH, Huang JS, Yang SF, Goo TS, Wang SH, Chen CL, Sanford TR (Jan 1991). "Acute poisoning with a glyphosate-surfactant herbicide ('Roundup'): a review of 93 cases". Human & Experimental Toxicology. 10 (1): 1–8. doi:10.1177/096032719101000101. PMID 1673618.
- ^ Romano RM, Romano MA, Bernardi MM, Furtado PV, Oliveira CA (Apr 2010). "Prepubertal exposure to commercial formulation of the herbicide glyphosate alters testosterone levels and testicular morphology". Archives of Toxicology. 84 (4): 309–17. doi:10.1007/s00204-009-0494-z. PMID 20012598.
- ^ United States Environmental Protection Agency (18 June 2007). "Draft List of Initial Pesticide Active Ingredients and Pesticide Inerts to be Considered for Screening under the Federal Food, Drug, and Cosmetic Act" (PDF). Federal Register. 72 (116): 33486–503.
- ^ United States Environmental Protection Agency (29 June 2015). "Memorandum: EDSP Weight of Evidence Conclusions on the Tier 1 Screening Assays for the List 1 Chemicals" (PDF).
- ^ ToxNet. Glyposate. National Library of Medicine.
- ^ a b András Székács and Béla Darvas. Forty years with glyphosate. In: Herbicides - Properties, Synthesis and Control of Weeds", Ed. Mohammed Naguib Abd El-Ghany Hasaneen, ISBN 978-953-307-803-8, Published: January 13, 2012.
- ^ "IARC monograph on glyphosate" (PDF). IARC. Retrieved September 27, 2015.
- ^ a b Solomon KR, Thompson DG (2003). "Ecological risk assessment for aquatic organisms from over-water uses of glyphosate". Journal of Toxicology and Environmental Health. Part B, Critical Reviews. 6 (3): 289–324. doi:10.1080/10937400306468. PMID 12746143.
- ^ Felix, F (2015). "Impact of the Herbicide Glyphosate Roundup (41%) on the Haematology of the Freshwater Fish Catla Catla (Hamilton)". Journal of Environmental Science, Toxicology, and Food Technology. 9 (4): 56–60.
{{cite journal}}:|access-date=requires|url=(help) - ^ NRA Special Review of Glyphosate (PDF). Canberra: Chemical Review Section, National Registration Authority for Agricultural and Veterinary Chemical, Australia. June 1996. Retrieved 5 September 2015.
- ^ do Carmo Langiano, V. and Martinez, C.B. (2008). "=Toxicity and effects of a glyphosate-based herbicide on the Neotropical fish Prochilodus lineatus". Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology. 147 (2): 222–231.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kelaniya, K.G. (2015). "Effect of glyphosate-based herbicide, Roundup™ on territory deference of male Oreochromis mossambicus (Osteichthyes, Cichlidae) associated with mating behaviour". Sri Lanka J. Aquat. Sci. 20 (1): 1–10.
- ^ a b Wagner N, Reichenbecher W, Teichmann H, Tappeser B, Lötters S (Aug 2013). "Questions concerning the potential impact of glyphosate-based herbicides on amphibians". Environmental Toxicology and Chemistry / SETAC. 32 (8): 1688–700. doi:10.1002/etc.2268. PMID 23637092.
- ^ "IARC monograph on glyphosate" (PDF). IARC. Retrieved September 27, 2015.
- ^ "Response to "The impact of insecticides and herbicides on the biodiversity and productivity of aquatic communities"" (PDF). Backgrounder. Monsanto Company. 2005-04-01.
- ^ "Aquatic Use of Glyphosate Herbicides in Australia" (PDF). Backgrounder. Monsanto Company. 2003-05-01.
- ^ Wojtaszek BF, Staznik B, Chartrand DT, Stephenson GR, Thompson DG (Apr 2004). "Effects of Vision herbicide on mortality, avoidance response, and growth of amphibian larvae in two forest wetlands". Environmental Toxicology and Chemistry / SETAC. 23 (4): 832–42. doi:10.1897/02-281. PMID 15095877.
- ^ Paganelli A, Gnazzo V, Acosta H, López SL, Carrasco AE (Oct 2010). "Glyphosate-based herbicides produce teratogenic effects on vertebrates by impairing retinoic acid signaling". Chemical Research in Toxicology. 23 (10): 1586–95. doi:10.1021/tx1001749. PMID 20695457.
- ^ Fernandez MR, Selles F, Gehl D, Depauw RM, Zentner RP (2005). "Crop Production Factors Associated with Fusarium Head Blight in Spring Wheat in Eastern Saskatchewan". Crop Science. 45 (5): 1908–16. doi:10.2135/cropsci2004.0197.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Duke SO, Wedge DE, Cerdeira AL, Matallo MB (2007). "Interactions of Synthetic Herbicides with Plant Disease and Microbial Herbicides". In Vurro M, Gressel J (eds.). Novel Biotechnologies for Biocontrol Agent Enhancement and Management. NATO Security through Science Series. pp. 277–96. doi:10.1007/978-1-4020-5799-1_15. ISBN 978-1-4020-5797-7.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Rosenblueth M, Martínez-Romero E (Aug 2006). "Bacterial endophytes and their interactions with hosts". Molecular Plant-Microbe Interactions. 19 (8): 827–37. doi:10.1094/MPMI-19-0827. PMID 16903349.
- ^ a b Lori (2009-05-07). "U of G Researchers Find Suspected Glyphosate-Resistant Weed". Uoguelph.ca. Retrieved 2010-08-22.
- ^ "Resisting Roundup". The New York Times. 2010-05-16.
- ^ Tarter S (2009-04-06). "PJStar.com". PJStar.com. Retrieved 2010-08-22.
- ^ ISU Weed Science Online – Are RR Weeds in Your Future I
- ^ Powles SB, Lorraine-Colwill DF, Dellow JJ, Preston C (1998). "Evolved Resistance to Glyphosate in Rigid Ryegrass (Lolium rigidum) in Australia". Weed Science. 46 (5): 604–7. JSTOR 4045968.
- ^ Glyphosate Resistance in Crops and Weeds: History, Development, and Management. Editor, Vijay K. Nandula. John Wiley & Sons, 2010 ISBN 9781118043547
- ^ "Glyphosate resistance is a reality that should scare some cotton growers into changing the way they do business". Southeastfarmpress.com. Retrieved 2010-08-22.
- ^ "Map of Glyphosate-Resistant Weeds Globally". The International Survey of Herbicide Resistant Weeds. 2010. Retrieved 12 Jan 2013.
- ^ Neuman W, Pollack A (4 May 2010). "U.S. Farmers Cope With Roundup-Resistant Weeds". New York Times. New York. pp. B1. Retrieved 4 May 2010.
- ^ Charles M Benbrook Impacts of genetically engineered crops on pesticide use in the U.S. - the first sixteen years Environmental Sciences Europe 2012, 24:24
- ^ Heap, I. The International Survey of Herbicide Resistant Weeds. Online. Accessed April 13, 2014 Resistance by Active Ingredient (select "glyphosate" from the pulldown menu)
- ^ "With BioDirect, Monsanto Hopes RNA Sprays Can Someday Deliver Drought Tolerance and Other Traits to Plants on Demand | MIT Technology Review". Retrieved 2015-08-31.
- ^ Culpepper AS, Grey TL, Vencill WK, Kichler JM, Webster TM, Brown SM, York AC Davis JW, Hanna WW (2006). "Glyphosate-resistant Palmer amaranth (Amaranthus palmeri ) confirmed in Georgia". Weed Science. 54 (4): 620–6. doi:10.1614/WS-06-001R.1. JSTOR 4539441.
{{cite journal}}: hair space character in|title=at position 57 (help)CS1 maint: multiple names: authors list (link) - ^ a b Hampton N. "Cotton versus the monster weed". Retrieved 2009-07-19.
- ^ a b Smith JT (March 2009). "Resistance a growing problem" (PDF). The Farmer Stockman. Retrieved 2009-07-19.
- ^ Taylor O (2009-07-16). "Peanuts: variable insects, variable weather, Roundup resistant Palmer in new state". PeanutFax. AgFax Media. Retrieved 2009-07-19.
- ^ Vargas L, Bianchi MA, Rizzardi MA, Agostinetto D, Dal Magro T (2007). "Buva (Conyza bonariensis) resistente ao glyphosate na região sul do Brasil". Planta Daninha (in Portuguese). 25 (3): 573–8. doi:10.1590/S0100-83582007000300017.
{{cite journal}}: Unknown parameter|trans_title=ignored (|trans-title=suggested) (help)CS1 maint: multiple names: authors list (link) - ^ Koger CH, Shaner DL, Henry WB, Nadler-Hassar T, Thomas WE, Wilcut JW (2005). "Assessment of two nondestructive assays for detecting glyphosate resistance in horseweed (Conyza canadensis)". Weed Science. 53 (4): 438–45. doi:10.1614/WS-05-010R. JSTOR 4047050.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ge X, d'Avignon DA, Ackerman JJ, Sammons RD (Apr 2010). "Rapid vacuolar sequestration: the horseweed glyphosate resistance mechanism". Pest Management Science. 66 (4): 345–8. doi:10.1002/ps.1911. PMC 3080097. PMID 20063320.
- ^ Vila-Aiub MM, Vidal RA, Balbi MC, Gundel PE, Trucco F, Ghersa CM (Apr 2008). "Glyphosate-resistant weeds of South American cropping systems: an overview". Pest Management Science. 64 (4): 366–71. doi:10.1002/ps.1488. PMID 18161884.
- ^ Jhala, Amit (4 June 2015). "Post-Emergence Herbicide Options for Glyphosate-Resistant Marestail in Corn and Soybean". CropWatch. Nebraska Extension. Retrieved 17 August 2015.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Preston C, Wakelin AM, Dolman FC, Bostamam Y, Boutsalis P (2009). "A Decade of Glyphosate-Resistant Lolium around the World: Mechanisms, Genes, Fitness, and Agronomic Management". Weed Science. 57 (4): 435–41. doi:10.1614/WS-08-181.1.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Vila-Aiub MM, Balbi MC, Gundel PE, Ghersa CM, Powles SB (2007). "Evolution of Glyphosate-Resistant Johnsongrass (Sorghum halepense) in Glyphosate-Resistant Soybean". Weed Science. 55 (6): 566–71. doi:10.1614/WS-07-053.1. JSTOR 4539618.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Pleasants, John M.; Oberhauser, Karen S. (2012). "Milkweed loss in agricultural fields because of herbicide use: effect on the monarch butterfly population" (PDF). Insect Conservation and Diversity. doi:10.1111/j.1752-4598.2012.00196.x.
- ^ "Occurrence of common milkweed (Asclepias syriaca) in cropland and adjacent areas". Crop Protection. 2000.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "NRDC Sues EPA Over Demise of Monarch Butterfly Population". NBC. 2015.
- ^ Staff, Centralamericadata.com. September 6, 2013 El Salvador: Use of 53 Chemicals Banned
- ^ Staff, Centralamericadata.com. November 27, 2013 El Salvador: Confirmation to Be Given on Ban of Agrochemicals
- ^ Legislative Assembly of El Salvador. November 26, 2013 Analizan observaciones del Ejecutivo al decreto que contiene la prohibición de 53 agroquímicos que dañan la salud English translation by Google
- ^ Staff, Sustainable Pulse. Apr 4 2014 Dutch Parliament Bans Glyphosate Herbicides for Non-Commercial Use
- ^ Staff, Colombo Page. May 22, 2015 Sri Lankan President orders to ban import of glyphosate with immediate effect
- ^ Sarina Locke for the Australian Broadcasting Corporation. Updated May 27, 2015 Toxicologist critical of 'dodgy science' in glyphosate bans
- ^ "HEALTH MINISTER: IMPORTATION OF ROUNDUP WEED SPRAY SUSPENDED". Bermuda Today. 11 May 2015. Retrieved 6 June 2015.
- ^ http://www.bbc.com/news/world-latin-america-32677411
- ^ Reuters.Jun 14, 2015 UPDATE 2-French minister asks shops to stop selling Monsanto Roundup weedkiller
- ^ Charry T (1997-05-29). "Monsanto recruits the horticulturist of the San Diego Zoo to pitch its popular herbicide". Business Day. New York Times.
- ^ "Monsanto fined in France for 'false' herbicide ads". Terradaily.com (January 26, 2007).
- ^ Europe | Monsanto guilty in 'false ad' row. BBC News (October 15, 2009).
- ^ a b "Testing Fraud: IBT and Craven Labs" (PDF). Backgrounder. Monsanto Company. June 2005.
- ^ Schneider K (Spring 1983). "Faking it The Case against Industrial Bio-Test Laboratories". The Amicus Journal. PlanetWaves.net: 14–26.
- ^ "EPA FY1994 Enforcement and Compliance Assurance Accomplishments Report" (PDF). United States Environmental Protection Agency.
- ^ Piller D (2010-04-01). "Albaugh accuses Chinese of dumping herbicide". Staff Blogs. Des Moines Register.
- ^ "In the Matter of: GLYPHOSATE FROM CHINA" (PDF). United States International Trade Commission. 2010-04-22.
- ^ Green JM, Owen MD (Jun 2011). "Herbicide-resistant crops: utilities and limitations for herbicide-resistant weed management". Journal of Agricultural and Food Chemistry. 59 (11): 5819–29. doi:10.1021/jf101286h. PMC 3105486. PMID 20586458.
- ^ Pollegioni L, Schonbrunn E, Siehl D (Aug 2011). "Molecular basis of glyphosate resistance-different approaches through protein engineering". The FEBS Journal. 278 (16): 2753–66. doi:10.1111/j.1742-4658.2011.08214.x. PMC 3145815. PMID 21668647.
- ^ Rashid A (2009). Introduction to Genetic Engineering of Crop Plants: Aims and Achievements. I K International. p. 259. ISBN 978-93-80026-16-9.
- ^ "Company History". Web Site. Monsanto Company.
- ^ "Adoption of Genetically Engineered Crops in the U.S." Economic Research Service. USDA. Retrieved 26 August 2015.