Why you should take this iterative approach to boost students’ understanding of electrochemistry across secondary and post-16 learning
Is electrochemistry one of the most difficult areas of secondary school chemistry study? Although some evidence-based research indicates that it may be, I’ve discovered in my teaching experience that electrochemistry doesn’t need to be a challenging topic if the curriculum follows a structured thread from 11–14 to post-16 study.
Is electrochemistry one of the most difficult areas of secondary school chemistry study? Although some evidence-based research (rsc.li/4c8Rgwt) indicates that it may be, I’ve discovered in my teaching experience that electrochemistry doesn’t need to be a challenging topic if the curriculum follows a structured thread from 11–14 to post-16 study.
Test recall
For students to develop a secure understanding, we need to follow a spiral curriculum that provides regular opportunities to build on and test the recall of prior learning. I see it as a thread that runs through the whole curriculum, and begins with atomic numbers in the first year of secondary school. Pupils will then need to understand electron configuration, and it’s during this part of the curriculum that you will recheck their understanding of atomic numbers and atomic structure.
Teach the electrolysis of molten compounds by treating it like the formation of ionic compounds in reverse
In my opinion, the spiral curriculum is essential for developing understanding of the production of halogens and hydrogen during the electrolysis of aqueous solutions. This would be a pointless exercise if students cannot demonstrate an understanding of diatomic gases and how ions form.
I have experienced the benefit of using retrieval practice, such as quizzes, throughout the curriculum. For example, I begin a lesson on half-equations at electrodes in electrolysis by revisiting the formation of ions from atoms studied earlier.
Build a solid foundation
My experience has shown that if students have a secure understanding of how atoms lose or gain an electron(s), it is so much easier to demonstrate how ions return to atoms with half-equations.
One of the misconceptions identified in the research cited at the beginning of this article was that some students believe electrons flow through a solution from one electrode to another. That’s why I like to begin with the story of electrolysis, focusing on the way anions lose electrons at the anode and then flow through wires to the cathode.
Similarly, if students understand that both ionic and covalent molecules can form ions in solution, they will no longer believe that electrolysis produces ions. One problem with this concept is that, up to GCSE, we teach students that only ionic compounds dissolve. Yes, they learn that acids dissociate when dissolved in water, but perhaps this is where the confusion lies.
Keep it simple
Often teachers omit practicals demonstrating that ions actually move towards the electrodes in post-16 electrochemistry teaching. But I always begin with a very simple set-up that shows the movement of manganate(VII) ions towards an anode from a small crystal sitting on a piece of filter paper wet with potassium nitrate solution (a salt bridge). Students can clearly see the direction of movement of these purple anions. Similarly, you can also demonstrate this with the blue colour of copper cations with a copper sulfate crystal.
Teach in reverse
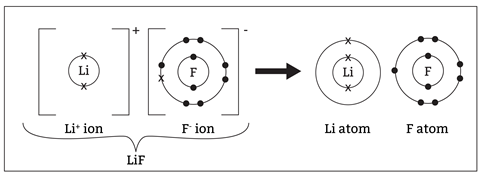
I prefer to teach the electrolysis of molten compounds by treating it like the formation of ionic compounds in reverse. Metals and non-metals tend to react to form more stable ions by the metal atom losing electron(s) and the non-metal gaining electron(s). This would usually be an exothermic process. If students have a secure understanding of this and they’ve revisited it, then the rest becomes logical. Electrolysis reverses this process by forcing stable ions to revert to less stable atoms. We require the chemical energy stored in the cell to drive this reversal.
Many of the issues I have addressed in this article will help your students progress in their understanding of electrochemistry. When I get my learners to revisit some of the essential prior knowledge in reverse I see the light bulbs go on in their heads and it becomes much easier for them to get electrochemistry.
Ian McDaid



![5 ideas a5000682 copper oxide and zinc reaction spl 300tb[1]](https://d1ymz67w5raq8g.cloudfront.net/Pictures/159x106/6/1/0/113610_5ideas_a5000682copper_oxide_and_zinc_reactionspl_300tb1.jpg)






No comments yet