Ensure students get to grips with the electrostatic forces between metal ions and delocalised electrons with these teaching ideas
What would you think was happening if all your buttons suddenly turned to dust? This is what happened to the soldiers in Napoleon’s army during their invasion into Russia over 200 years ago. And materials scientists believe it’s because the buttons on the soldiers’ uniforms that held their trousers up, and kept their coats closed, were tin. At temperatures below 13°C, the structure of tin starts to change. It becomes brittle and crumbly, and its electrons cannot move freely. Although the process is very slow even at -10°C, the transition becomes much quicker when temperatures of -33°C are quite common. Maybe the French army thought that it was a Russian defence tactic?
What students need to know
At 11–14 students learn that metals are elements found on the left of the periodic table. They will be able to describe the properties of metals. They will understand that metals conduct electricity owing to the free-moving, negatively-charged particles called electrons that carry electrical charge from one end of the metal to the other when you apply a potential difference.
At 14–16, learners need to know:
- Metals consist of giant structures of close-packed metal ions arranged in a regular pattern surrounded by delocalised electrons.
- The layers of ions can slide over each other, which allows metals to be bent and shaped.
- A metallic bond is the electrostatic force of attraction between the metal ions and the delocalised electrons that extend in all directions.
- The strength of metallic bonding means most metals have high melting and boiling points.
At 14–16, learners need to know:
- Metals consist of giant structures of close-packed metal ions arranged in a regular pattern surrounded by delocalised electrons.
- The layers of ions can slide over each other, which allows metals to be bent and shaped.
- A metallic bond is the electrostatic force of attraction between the metal ions and the delocalised electrons that extend in all directions.
- The strength of metallic bonding means most metals have high melting and boiling points.
To understand the structure and bonding in metals students must first have a good understanding of atomic structure and be familiar with the concept of opposite charges attracting.
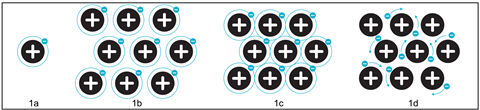
Build up the structure of a metal in the solid state by using atoms drawn on transparent acetates that you can easily move under a visualiser. Start with a simple model of an atom showing the outer shell electrons like in Figure 1a. Importantly at this point, unpick what the solid black sphere represents – the nucleus and inner shell electrons. Arrange several atoms in a regular array, as shown in Figure 1b and then slowly pack the atoms closer and closer until the outer shells overlap, as in Figure 1c. Once close-packed, students will be able to see that the outer shell electrons are now free to move through the structure. Finally introduce the idea that the electron shells are artificial constraints and that the electrons can drift freely without set paths (Figure 1d). Introduce the term delocalised by asking students what it means to be local to the area.
Common misconceptions
The misconceptions that arise within this topic are a result of the models, such as the one described here, and the associated terminology that we use to describe metallic bonding. Many resources refer to metals as regularly arranged metal atoms surrounded by a sea of delocalised electrons. Others state that the structure consists of metal ions. Some refer to an atomic core.
The correct description depends on whether we consider the metal atom to have fully lost an electron. Although in a metallic structure the electron is no longer attached to one particular atom, it is still present in the structure. The formula for a metal is Na and not Na+, for example. What is definite is the black sphere in the model described in Figure 1 is positively charged. Be upfront with students regarding the different terminology and models used and ask them which they think is most appropriate.
Suggestions for teaching
- Tackle misconceptions head-on by discussing the limitations of the model we use to describe the bonding in metals.
- Make links between the different types of bonding (ionic, covalent, metallic) by focusing on the electrostatic forces involved.
- Compare the bonding in a metal to that in an ionic compound to avoid learners confusing ionic and metallic bonding diagrams.
The sea metaphor for the delocalised electrons can also influence students to the extent that they draw diagrams of a sea consisting of a vast excess of electrons. This misconception can be exacerbated by drawings showing small metal ions surrounded by large amounts of white space. You can use the diagnostic questions and response activities for metallic structure diagrams within the Best Evidence Science Teaching collection to unpick students’ understanding and tackle misconceptions head-on.
The sea metaphor for the delocalised electrons can also influence students to the extent that they draw diagrams of a sea consisting of a vast excess of electrons. This misconception can be exacerbated by drawings showing small metal ions surrounded by large amounts of white space. You can use the diagnostic questions and response activities for metallic structure diagrams within the Best Evidence Science Teaching collection (bit.ly/3LWot6E) to unpick students’ understanding and tackle misconceptions head-on.
Checking for understanding
Once learners understand the model, explaining the properties of a metal is something most students find relatively straightforward.
Metals have high melting points because of the strong electrostatic forces of attraction between the positively charged metal ions and the delocalised electrons. At A-level, you can extend this understanding to explain trends in melting points down group 1 or across period 3. Students can also investigate the different ways the atoms in a metal can pack closely by making physical models using spheres, such as marbles. Encourage learners to evaluate these models to stimulate discussions about the difficulty of developing models for bonding. Unlike spherical balls, atoms and ions do not have clearly defined boundaries so how realistic is the model?
To students, the bonding in a metal can look very similar to the bonding in an ionic compound. Both consist of a lattice of charged particles held together by electrostatic forces of attraction, yet metals are malleable and ionic salts are brittle. Ask students to compare the two structures and explain these differences in properties.
Ideas for your classroom
Tackle misconceptions by discussing the limitations of the model we use to describe bonding in a metal. Make links between different types of bonding (ionic, covalent, metallic) by focussing on the electrostatic forces involved. Take care to compare the bonding in a metal to that in an ionic compound to avoid learners confusing ionic and metallic bonding diagrams.
More resources
- Explore students’ understanding of the bonding in metals and highlight any misconceptions by using the true/false statements in Chemical misconceptions II: iron – a metal.
- Use our Metallic bonding structure strips with your 14–16 year-old learners to help them retrieve information on the metallic bonding model and explain how this leads to particular properties.
- Identify learning gaps and misconceptions relating to bonding with these Review my learning worksheets.
- Watch the Properties of alloys: finding the NaK demonstration to extend students’ understanding of metallic bonding and the particle model of matter.
- Highlight careers that use chemistry, such as Lucia’s role as a museum scientist where she analyses objects to help the Victoria and Albert Museum curators and conservators.
More resources
- Solidify students’ understanding of the structure and properties of metals and alloys with this poster, fact sheet and scaffolded student worksheet: rsc.li/4dTrsbV
- Use our metallic bonding structure strips with your 14–16 year-old learners to help them retrieve information on the metallic bonding model and explain how this leads to particular properties: rsc.li/4d6fEmO
- Identify learning gaps and misconceptions relating to bonding with these Review my learning worksheets: rsc.li/4fBNm5w
- Watch the Exhibition chemistry demonstration ’Properties of alloys: finding the NaK’ to extend students’ understanding of metallic bonding and the particle model of matter: rsc.li/4cehBw7
- Highlight careers that use chemistry, such as Lucia’s role as a museum scientist where she analyses objects to help the Victoria and Albert Museum curators and conservators: rsc.li/3X3aR05
Catherine Smith is chemistry lead at The Hinckley School, an 11–18 academy in Leicestershire











1 Reader's comment