Abstract
Corneal blindness is the major cause of vision impairment and the fourth-largest leading cause of blindness worldwide. An allograft corneal transplant is the most routine treatment for visual loss. Further complications can occur, such as transplant rejection, astigmatism, glaucoma, uveitis, retinal detachment, corneal ulceration due to reopening of the surgical wounds, and infection. For patients with autoimmune disorders, allografting for chemical burns and infections is contraindicated because of the risk of disease transmission and further complications. Moreover, corrective eye surgery renders the corneas unsuitable for allografting, further increasing the gap between donor tissue demand and supply. Due to these challenges, other therapeutic strategies such as artificial alternatives to donor corneal tissue are being considered. This review focuses on the use of alginate as a building block of therapeutic drugs or cell delivery systems to enhance drug retention and encourage corneal regeneration. The similarity of alginate hydrogel water content to native corneal tissue makes it a promising support structure. Alginate possess desired drug carrier characteristics, such as mucoadhesiveness and penetration enhancing properties. Whilst alginates have been extensively studied for their application in tissue engineering (TE), with many reviews being published, no reviews exist to our knowledge directly looking at alginates for corneal applications. The role of alginate in drug delivery to the surface of the eye and as a support structure (bioinspired tissue scaffold) for corneal TE is discussed. Biofabrication techniques such as gel casting, electrospinning, and bioprinting to develop tissue precursors and substitutes are compared. Finally, cell and tissue encapsulation in alginate for storage and transport to expand the scope of cell-based therapy for corneal blindness is also discussed in the light of recent applications of alginate in maintaining the function of biofabricated constructs for storage and transport.
Export citation and abstract BibTeX RIS
Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The eye is a complex organ and, compared with most other human organs, provides easy access for medical observation and surgical manipulation (Chirila and Harkin 2016). According to the World Health Organization (WHO), corneal blindness is the major cause of vision impairment and the fourth-largest leading cause of blindness worldwide, followed by cataracts. Pathological conditions affecting the cornea account for 4.9 million cases of blindness worldwide. The main causes of corneal diseases and blindness are described in more detail in the subsequent section. An allograft corneal transplant is the most routine treatment for visual loss. To this date there are two main options to address the growing need for corneal translplants available. One of them being an allograft from a human donor, which has drawbacks due to the limited availability of quality donor graft material and potential graft rejection or a corneal tissue engineered alternative created out of synthetic or natural polymers (Ghezzi et al 2015).
The field of corneal tissue engineering (TE) has advanced considerably in the recent years. Yet the challenges associated with engineering a mechanically stable, optically transparent and biocompatible alternative that aids in regeneration still remain. To address these challenges the main approaches that are employed in the field of corneal TE are: cell-based stategies that rely on stimulating the cells to produce extracellular matrix (ECM) to support them, and scaffold based alternatives that act as structually stable, transparent ECM upon which the cells can grow. Both strategies have their advantages and have met with some degree of success (reviewed in Shah et al 2008). Artificial alternatives to donor corneal tissue are attracting great interest. Different methods ranging from keratoprosthetics to regenerative medicine, whereby the native cornea is stimulated by means of an implant to produce a wound-healing response, have been developed (Polisetti et al 2013).
This review focuses on the use of alginate as a building block of therapeutic drugs or cell delivery systems to enhance drug retention and encourage corneal regeneration. Alginate hydrogels are frequently chosen to act as support structures for corneal TE, due to the similarity of the hydrogel water content to native corneal tissue. Additionally, alginate has the desired drug carrier characteristics: it is a good penetration enhancer with high mucoadhesive strength and can transition from sol (liquid) to gel once instilled in the cul-de-sac of the eye (Lin and Sung 2000, Khare et al 2014). Other benefits of alginate include non-cytotoxicity, high biocompatibility, good construct stability, rapid gelation, low cost, and transparency. These beneficial attributes of alginate justify the selection of the polymer as the core of this article. The review covers new approaches to introducing drug-laden composite alginate hydrogels into the eye surface and introducing nanoparticle (NP)-encapsulated drugs added to alginate gel into the eye. In addition, the role of alginate as a support structure (bioinspired tissue scaffold) for corneal TE is discussed. Biofabrication techniques such as gel casting, electrospinning, and bioprinting to develop tissue precursors and substitutes are compared. Finally, cell and tissue encapsulation in alginate for storage and transport to expand the scope of cell-based therapy for corneal blindness is also discussed in the light of recent applications of alginate in maintaining the function of biofabricated constructs for storage and transport.
1.1. Diseases of the cornea
Corneal diseases are primarily caused by infectious agents and the accompanying degeneration and corneal dystrophy, either hereditary or environmental, such as Stephen–Johnson syndrome, keratoconus, and Fuch's endothelial dystrophy (Iqbal et al 2019). Stephen–Johnson syndrome is a rare and serious disorder that occurs due to a severe adverse response to medication or an infection and affects the skin and mucous membranes. It involves multiple ocular problems such as conjunctivitis, iritis (ocular inflammation), corneal fibrosis, blisters and perforation, conjunctival scarring, and, in acute phases, corneal neovascularization and complete opacification. These complications can lead to permanent vision loss. Keratoconus is a non-inflammatory condition characterized by thinning and bulging of the cornea, affecting the ability of the eye to focus and leading to blurred vision, corneal scarring, or, in severe cases, complete vision loss, which requires a corneal transplant, which is considered the gold-standard treatment. Fuch's endothelial dystrophy is characterized by corneal oedema (accumulation of fluid within the cornea) and thickening due to the destruction or loss of function of corneal endothelial cells responsible for maintaining the fluid equilibrium within the cornea. Disease progression affects the epithelial layer, which can rupture due to corneal swelling, causing painful corneal abrasions and poor vision; this case usually requires a corneal transplant. Other conditions affecting the cornea that can lead to corneal opacification and subsequently require a corneal transplant include limbal stem cell deficiency (LSCD), which leads to a lack of corneal epithelial repopulation. In addition, chemical burns, a penetrating eye injury, post-operative complications, viral infections (e.g. ocular herpes caused by herpes simplex virus II), keratitis due to bacterial or fungal infections, severe cases of dry eye, and infections associated with injury from wearing contact lenses can all cause corneal ulceration, opacification, and poor vision.
1.2. Structure of the cornea
The cornea is a dome-shaped, transparent avascular tissue located at the anterior part of the eye. It comprises three cellular layers (epithelium, stroma, and endothelium), which are separated by Bowman's and Descemet's acellular layers (Matthyssen et al 2018). The cornea acts as a structural barrier, protecting the eye against foreign bodies, and contributes to two-thirds of the refractive power of the eye.
The corneal epithelial layer is a stratified squamous non-keratinized epithelium at the anterior of the cornea. The cornea is 540–560 µm thick, and multiple desmosomes connect adjacent cells together and to the underlying Bowman's membrane (Forrester et al 2016). If a corneal abrasion or scratch occurs, epithelial cells undergo continuous mitosis, rapidly regenerating the epithelial layer. Epithelial cells have the highest proliferative capacity, followed by stromal keratocytes and endothelial cells, which are the least renewable. Stromal keratocytes are the main cell type found in the lamellae of the stroma. They are modified fibroblasts, have a stellate appearance with thin cytoplasmic extensions, and have a density of 20 000–24 000 cells mm−2 throughout the stroma, connecting to their neighboring cells via gap junctions (Forrester et al 2016, Nosrati et al 2020).
The endothelium, the posterior surface of the cornea, is composed of a simple squamous endothelium that is critical in maintaining corneal hydration and, subsequently, transparency. Endothelial cells are 5–6 µm high and 18–20 µm wide and form an uninterrupted hexagonal array below the Descemet's membrane (Forrester et al 2016). Their regenerative capacity in the native human cornea is believed to be low; however, there is some evidence of the presence of putative endothelial stem cell (SC) niches in the extreme peripheral cornea. Upon damage and a reduction in the endothelial cell number to <800 cells mm−2, oedema, stromal swelling, and subsequent loss of corneal transparency occur, causing decreased vision and pain. Mechanical (e.g. pseudophakic bullous keratopathy) or age-related trauma (e.g. Fuch's endothelial dystrophy) can cause abnormal endothelial cell death, requiring a corneal transplant and stressing the importance of finding artificial corneal substitutes (Matthyssen et al 2018, Tsai and Daniels 2021). Other corneal cell types include Langerhans and dendritic bone-marrow-derived immune cells, trigeminal nerve dendrites, Schwann cells, and histiocytes (Forrester et al 2016).
The stroma is a dense connective tissue predominantly composed of water (78%) and makes up 90% of the corneal thickness. It mainly consists of tightly interwoven 200–250 layers of 2 µm-thick, 30 nm-diameter lamellae (flattened collagen fibrils) arranged orthogonally throughout the center of the cornea. The narrow diameter of these flattened collagen fibrils that make up a lamella is an important factor contributing to corneal transparency. The density of the lamellae varies between the anterior lamellae and the posterior, with the latter being less densely arranged (Bergmanson et al 2005). This strictly regular geometric organization of lamellae maintains the overall shape of the cornea and contributes to corneal birefringence. The lamellae consist largely of type I collagen fibrils; types III, V, and VI are also present. Corneal transparency at the microscopic level is achieved by the absence of scatter and changes in refractive index by the corneal cells. On the nanoscopic level transparency is achieved by the structure and organization of collagen fibrils within the lamellae, as well as the absence of pigments and blood vessels (Meek and Knupp 2015). Glycosaminoglycans (mainly keratin sulfate and chondroitin) and proteoglycans connect the collagen fibers. However, injuries or infection activate native repair mechanisms, resulting in additional irregular collagen deposition and thus leading to scarring and opacity of the cornea and subsequent loss of function.
1.3. Regenerative therapies for corneal restoration
An allograft corneal transplant is the most routine treatment for visual loss due to a lack of transparency. Replacement of the entire cornea is called penetrating keratoplasty, while partial replacement of the cornea is called lamellar keratoplasty. Approximately 185 000 corneal transplants (the second-most common transplant after blood donation) are performed worldwide annually, yet ∼12.7 million people are estimated to be on the waiting list for corneal transplantation worldwide (Gain et al 2016). In the UK alone, there is an estimated shortage of 1500 corneas per year (Gaum et al 2012). In addition, even after corneal transplantation, further complications can occur, such as transplant rejection, astigmatism, glaucoma, uveitis (inflammation of the middle layer of the eye), retinal detachment, corneal ulceration due to reopening of the surgical wounds, and infection. Unfortunately, for patients with autoimmune disorders, allografting for chemical burns and infections is contraindicated because of the risk of disease transmission and further complications (Polisetti et al 2013). Moreover, corrective eye surgery renders the corneas unsuitable for allografting, further increasing the gap between donor tissue demand and supply. Due to these challenges in corneal transplantation, other therapeutic strategies are being considered. Currently available regenerative therapies include conjunctival limbal autografting (CLAU), which is transplantation of a graft harvested from the limbus of the patient's healthy eye (Kenyon and Tseng 1989); cultivated limbal epithelial transplantation (CLET), which is transplantation of limbal cell sheets cultured from limbal biopsies (Pellegrini et al 1997); and, the most recent, simple limbal epithelial transplantation (SLET), which combines the benefits of both CLAU and CLET without their limitations. The ex vivo cultivation necessary for CLET is considered superfluous and expensive, while autografting technically is considered challenging and risky. In SLET, a small biopsy is taken from the limbus of the patient's healthy eye and divided and distributed over the human amniotic membrane, which is then transplanted onto the affected cornea. Each small explant promotes re-epithelization over the course of 2 weeks. SLET restored the corneal epithelium in 83% of procedures. Visual acuity improved in 69% of reported cases (Sangwan et al 2012). SLET is associated with reduced risk of iatrogenic LSCD developing in the healthy eye due to the biopsy. In addition, due to direct transfer of limbal epithelial cells, there is no need for expensive ex vivo cell expansion in a specialized laboratory. The ability to perform the biopsy and the transplant in one operation makes SLET more convenient for both the surgeon and the patient (Sangwan et al 2012, Jackson et al 2020). If autologous transplantation is not possible, keratolimbal allograft (KLAL) can be performed, in which the tissue is harvested from a cadaveric donor, which requires a living related donor or systemic immunosuppression with cyclosporin A prior to transplant to in order prevent graft rejection. Corneal graft clarity and visual improvement after KLAL have been reported during follow-up; however, incidents of rejection or LSCD recurrence have also been noted (Ozer et al 2020).
In 2014, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) authorized the sale of Holoclar. Holoclar is a SC based treatment developed by the Italian company Holostem as a regenerative medicine for the eye. This is the first innovative therapy containing SCs, indicated for the treatment of moderate-to-severe keratitis due to severe LSCD (caused by chemical eye burns). A currently ongoing multinational, multicentre, open-label, uncontrolled clinical trial to assess the efficacy and safety of the procedure for restoration of corneal epithelium in patients with LSCD due to ocular burns has been established. Based on the evidence of quality and control of the manufacturing process, safety, efficacy and on a positive benefit–risk balance in 104 (72.1%) of 148 patients treated, Holoclar received conditional Marketing Approval in the EU (Sikora et al 2019). Holoclar is the first medicine approved in the EU for LSCD—a rare condition that can result in blindness. Holoclar comprises ex vivo expanded autologous human corneal epithelial cells containing SCs and is classified as 'tissue engineered product'. The role of limbal SCs is to renew the outer cells of the cornea. Holoclar, designated an orphan drug, is used to replace damaged limbal SCs on the corneal surface. The patient's limbal cells are removed from the edge of the cornea, amplified, and then transplanted onto the damaged corneal surface.
The therapeutic goal is to replace the corneal epithelium and limbal SCs (Ghareeb et al 2020). The aim of corneal repair is for the implanted SCs to (a) multiply, differentiate, and migrate to regenerate the corneal epithelium and (b) maintain a reservoir of SCs that can continuously regenerate the corneal epithelium. Each Holoclar preparation contains one dose of treatment with a sufficient number of cells to cover the entire corneal surface. The recommended dose is 80 000–300 000 cells cm−2, corresponding to 1 cm2 of product/cm2 of lesion.
SCs from a biopsy sample of 1–2 mm2 of undamaged limbal tissue from the patient are amplified ex vivo. The biopsy sample is collected under local anesthesia. Briefly, after washing the ocular surface with sterile balanced salt solution, the conjunctiva is detached from the limbus to expose the corneal site of sampling. A 2 × 2 mm2 incision is made for the biopsy, which is sent to the manufacturer within 24 h of collection. Topical antibiotic prophylaxis is performed after collection. Holoclar is exclusively intended for the regeneration of autologous limbal SCs, in compliance with the approved therapeutic indication. It needs to be implanted under aseptic conditions. A limbal peritomy, a conjunctival incision and excision of the corneal fibrovascular tissue from the defective area, is performed. Next, the implant is put in place under the incised conjunctiva, any excess part is cut away, and the edges are covered by the conjunctiva and sewn with three stitches, forming a physical seal on the lesion and securing the implant. Topical, systemic anti-inflammatory and antibiotic prophylaxis is performed post-implantation (Arrighi 2018).
Despite these advancements in regenerative therapies for corneal restoration, there is still shortage of good-quality donor tissue, emphasizing the need for artificial alternatives.
2. Corneal tissue engineering
TE has been described as 'the persuasion of the body to heal itself, through the delivery to the appropriate sites of molecular signals, cells and supporting structures' (Chirila and Harkin 2016). TE aims to recapitulate the native tissue morphology, compensating for the loss or failure of organs by developing functioning alternatives. Corneal TE is a practical strategy for developing corneal tissue alternatives. For corneal TE, it is paramount to consider the design, mechanical properties, and transparency of the scaffold, mimicking the natural cornea (Kong and Mi 2016).
2.1. Biomaterials for ocular tissue engineering
The first successful transplantation of donor tissue into a human was carried out by Eduard Zirm in 1906. A successful bilateral corneal transplant was performed on a 45 year-old farmer with lime burns. The donor was an 11 year-old boy with penetrating eye injury, whose corneas were enucleated and then stored in a warm physiological saline solution. The graft was sufficiently functional to allow the farmer to return to lighter agricultural work at home. Zirm attributed his success to the good nutritional condition of the corneas from a young patient as well as the perfect fit of the transplant (Armitage et al 2006). Prior to this, in 1862, external materials were implanted for the first time into the eye, with the aim of creating biomaterials (Chirila and Harkin 2016). Italian ophthalmologist Onofrio Abbate experimentally maintained a keratoprosthesis in animal corneas. A glass disk was encased in a skirt of two successive rings. Abbate used natural polymers for both rings: the first ring was made of gutta-percha, also known as caoutchouc, which is a trans-polyisoprene derived from the exudate of trees. The second ring was made of casein, a common protein found in milk products that can be obtained by precipitation of milk or cheese. The rings were meant to promote bio integration of the construct; however, Abbate did not account for the gutta-percha and casein becoming brittle on exposure to air and light. Abbate's first artificial corneal equivalent transplantation resulted in a rejection by the host within a week. These findings demonstrate how important it is to determine and consider the integrity (such as stability upon exposure to air and light) of implanted biomaterials at different time points because it will ultimately determine clinical success, which is driven by the host response to the biomaterial post implantation (Badylak and Anderson 2015).
Due to severe corneal donor shortages, regenerative strategies using biomaterials with or without cells have become popular (Matthyssen et al 2018). Biomaterials are engineered substances that are interfaced with biological systems to diagnose, treat, augment, or restore any tissue, organ, or function in the body (Liu et al 2006). Biomaterials can also refer to living substances or materials of natural origin (Chirila and Harkin 2016). Biomaterials emulate natural tissue. Their physical, chemical, and biological properties can be modified depending on the biological environment of the host tissue being recapitulated and the expected function post-implantation in the host, so they are an attractive alternative to native tissue. Biomaterial design and selection are crucial for TE. The factors to be considered when selecting a suitable biomaterial include (a) favorable integration of the biomaterial with the host cells and (b) its ability to stimulate regeneration by inducing cell signaling cascades that lead to the production of growth factors necessary for cell proliferation, cell differentiation, and the deposition of the supporting ECM (Iqbal et al 2019). The ideal biomaterial facilitates cell or transforming factor localization to the desired sites in the body and guides the development or repair of new tissues with appropriate function. The biomaterial needs to preserve the bioactivity of the drug, protecting its structure from denaturation, pH changes or enzymatic digestion, be able to support a large payload of the drug in a minimal volume, delivery needs to be targeted, specific, biocompatible and avoid raising intraocular pressure (IOP) during administration. Upon delivery, sustained release of the biomolecule should be maintained for a prolonged period of time to avoid frequent administration and improve patient compliance. In addition, the biomaterial must be able to act as an artificial ECM; the scaffold should match the mechanical and biological properties of the native tissue. Degradation products of biomaterials should not induce an immune response in the host and should be successfully removed from the body via metabolic pathways (Iqbal et al 2019). Ophthalmic biomaterials have a unique prerequisite—that of being transparent. Historically, glass and quartz have been used for contact lenses; however, polymers have gradually become the ophthalmic material of choice. The adjustable nature as well as the transparency of synthetic polymers, biopolymers, and their combination (hybrid materials) make them attractive for ophthalmic applications (Chirila and Harkin 2016).
To meet the requirements for clinical use, multiple techniques are used. Fabrication of different compositions of scaffolds depends on the control of the polymers' molecular weight, polydispersity (a measure of the heterogeneity of sizes of molecules or particles in a mixture), crystallinity (the degree of structural order in a solid), thermal transition (molecular transitioning from one phase to another due to the addition of thermal energy), and degradation rate (Iqbal et al
2019). Modifying these parameters allows for high structural precision during the assembly stages controlling the degradation, porosity, and stiffness of the scaffolds produced and strongly affects their properties (Gomes and Reis 2004). In medical applications, synthetic polymers are normally favored because of their high manufacturing reproducibility compared to natural polymers and limited batch-to-batch variability (Nair and Laurencin 2007). The first synthetic polymer to be implanted in the vitreous cavity was poly-1-vinyl-2-pyrrolidinone (Hong et al
1996, Chirila and Harkin 2016). Subsequently, more than 300 potential candidates were identified, and preliminary selection was based on their mechanical and optical properties in vitro; only 12 of 300 were suitable for further evaluation, stressing the difficulty and rigorous testing required when developing ophthalmic biomaterials (Hong et al
1996, Chirila and Harkin 2016). Currently, the most frequently used synthetic polymers for TE and drug delivery are aliphatic polymers (e.g. poly-lactic acid, poly-glycolic acid, poly-lactic-co-glycolide, poly--caprolactone, poly-p-dioxanone), plus copolymers soft trimethylene carbonate and glycolide. Other biodegradable synthetic polymers include polyanhydrides, polyphosphazenes, polyurethanes, poly-glycerolsebacate, synthetic hydrogels, and functional synthetic polymers (Shin et al
2003).
On their own, synthetic hydrogels do not support cell adhesion; however, they can be chemically modified to suit specific applications (Bajaj et al 2014, Murphy et al 2020) and functionalized with ECM proteins to facilitate cell support.
Natural biopolymers include polysaccharides, proteins, and polyesters derived from both plants and animals, such as starch, alginate, chitin/chitosan, hyaluronic acid derivatives, collagen, fibrin, and silk (Kumbar et al 2014, Mirazul Islam et al 2015, Arica et al 2021). Natural hydrophilic polymers can form hydrogels due to the presence of hydrophillic functional groups that are attached to the backbone of the polymer, which absorb large amounts of water. Crosslinks between network chains are formed which creates the resistance to dissolution (Ahmed 2015). The hydrated network structure creates a favorable environment for the encapsulation of cells and other biomaterials, and due to their low cytotoxicity and structural similarity to the native ECM, natural hydrophilic polymers are commonly used as scaffolds in TE applications and as bioinks in 3D bioprinting (Lee and Mooney 2012, Kyburz and Anseth 2015). The inherent biocompatibility of some natural polysaccharides (e.g. collagen) allows them to form a template for cell attachment and proliferation; however, the risk of inducing an immune response in the host exists. The molecular structure of natural polymers is highly organized, containing extracellular ligands that can bind to cell receptors. The degradation rate of both natural and synthetic polymers is different from patient to patient because the degradation of natural polymers depends on patient enzyme activity, which varies (Mirazul Islam et al 2015). Polymers isolated from other non-mammalian sources, such as alginate, are also common (Bajaj et al 2014).
3. Alginate
Alginate is a linear anionic polysaccharide composed of β-D-mannuronic acid (M block) and α-L-glucuronic acid (G block) blocks (Bishop et al 2017). The blocks can be made up of consecutive G residues (GGGGGG), consecutive M residues (MMMMM), or alternate M and G residues (GMGMGMG). Alginate is typically obtained by treating the cell walls of brown algae of the class Phaeophyceae with sodium hydroxide (NaOH). Upon filtration, sodium chloride or calcium chloride is added to the solution, and then the mixture is treated with diluted HCl, forming sodium alginate or calcium alginate, respectively. Figure 1 illustrates the species that can yield alginate, which include Laminaria hyperborea, Laminaria digitata, Laminaria japonica, Ascophyllum nodosum, and Macrocystispyrifer (Lee and Mooney 2012).
Figure 1. Composition and structure of different alginates obtained from brown seaweeds.
Download figure:
Standard image High-resolution imageThe M and G contents and length of the alginate polymer extracted from different sources vary. The M:G block ratio affects the physiochemical properties of alginate. Alginate with a higher M block ratio is more flexible and elastic compared with alginate with a higher G block ratio, which is rigid and stiff (Lee and Mooney 2012). X-ray studies have determined that alginates with higher G content have smaller spacings along the polymer axis (8.72 Å) compared with alginates with higher M content (10.35 Å). The stereochemical arrangement of the uronic acid unit conformation supports these findings, as the MM block is di-equatorially linked at C-1 and C-4, creating a flat, ribbon-like, straight polymer chain, while the GG block is formed from diaxial groups at both C-1 and C-4, forming a bucked polymer chain (Penman and Sanderson 1972, Atkins et al 1973, Qin 2008). These differences in arrangement affect the physiochemical properties of alginate when in contact with crosslinking agents, as the formation of a stronger gel mainly involves the GG block. Alginate is water soluble and can rapidly gel by crosslinking with divalent cations such as calcium, strontium, and barium. Crosslinking is achieved when the carboxyl group of α-l-guluronic acid encounters a divalent cation such as calcium chloride, forming an egg-box model (Lee and Mooney 2012). Crosslinking occurs by either directly depositing alginate into a pool of the crosslinking solution or spraying it over an extruded cell-laden hydrogel. In addition, alginate is a highly extrudable material that can be used in 3D bioprinting, a promising TE technique in regenerative medicine. Ease of gelation makes alginate an interesting candidate for use in TE as well in 3D printing as a bioink. Alginate also shows good biocompatibility, low cytotoxicity, non-immunogenicity, and the ability to be functionalized with other components to improve cellular adhesion and proliferation (alginate lacks cell-adhesive moieties and is bioinert). This last property can be an advantage if the proliferation of encapsulated cells needs to be arrested. The disadvantages of alginate can be overcome by chemically modifying it with bioactive materials, such as proteins or RGD motifs, to encourage cellular adhesion and cell–matrix interaction.
Viscosity is another important parameter to consider when designing scaffolds. In alginate hydrogels, viscosity depends on the polymer's molecular weight and the percentage concentration. Additionally, the phenotype of cells used for loading can affect the final viscosity (Bishop et al 2017). Higher-viscosity alginate restricts the encapsulated cells' ability to proliferate and migrate, while lower-viscosity alginate facilitates cell viability and propagation. The mechanical strength of low alginate concentrations decreases; therefore, it is important to optimize the density to retain both cell viability and construct stability.
Another advantageous material property of alginate is muco-adhesiveness, which makes it a good penetration enhancer able to increase the pre-corneal residence of a drug at the ocular site. Muco-adhesiveness can be described as the adhesion of a drug delivery system to the mucosal surface with the purpose of releasing the encapsulated drug in a controlled manner. Mucus forms the innermost layer of the tear film, adheres to the mucosal epithelial surface and is responsible for the formation of the adhesive interface. Goblet cells that are generally closely associated with glycocalyx of the corneal or conjunctival epithelial cells are responsible for the production of mucins that coat the ocular surface (Gipson et al 1992). The main function of mucus is to facilitate the movement of the eyelids, entrap bacteria and other debris, enhance stability and cohesion of the tear film, and protect the epithelial cells from damage (Greaves and Wilson 1993, Khare et al 2014). Alginate is anionic with multiple hydrophilic carboxyl end groups, which cause the polymers to swell when hydrated and expose adhesive sites. Subsequently, entanglement and or interpenetration of the polymer chains with the mucin chains occurs. This establishes physio-chemical interactions in a form of hydrogen bonding and electrostatic forces between the mucoadhesive polymer and the glycoprotein mucin network covering the corneal epithelium. Additionally, the difference in surface tension between alginate (31.5 Mn m−1) and the mucin coating (38 mN m−1) contributes to good muco-adhesiveness and spreading of the polymer is beneficial in treating severe corneal diseases by enhancing penetration and increasing the precorneal residence of the drug at the ocular site (Mittal 1977, Khare et al 2014).
Although overall, alginate is negatively charged and generally positively charged materials provoke an inflammatory response, alginates with high M block ratios can be more immunogenic compared to alginates with a higher G block ratio (Kulseng et al 1999, Axpe and Oyen 2016). Figure 1 shows the structural composition of alginates obtained from brown seaweeds.
Although high-molecular-weight alginate has a slow, uncontrollable degradation rate, sodium periodate oxidation of alginate alters its backbone confirmation, allowing it to degrade in a prescribed manner, facilitating more ready clearance of alginate from the host body (Bouhadir et al 2001). This is an advantageous property of alginate for use in drug delivery and cell transplantation. Moreover, oxidized alginate gels serve as viable corneal wound-healing bandages, which not only provide a hydrated environment to the corneal surface but also encourage corneal re-epithelization (Wright et al 2013).
The use of alginate as a biomaterial in corneal regeneration and TE will be discussed next.
4. Alginate in corneal tissue engineering
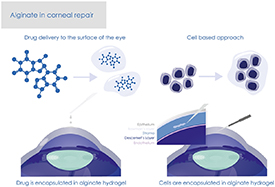
The two main TE approaches are a cell-based methodology, whereby cells in a permissive environment are the driving force for construct TE via the deposition of ECM components, and a scaffold-based methodology, in which focus is on developing a material substrate that mimics the ECM microenvironment providing support to the cell population of interest. The scaffold-based approach is more prevalent in the development of biomimetic corneal replacements using (a) crosslinked collagen scaffolds, mimicking the natural corneal stroma, or (b) a wide range of other materials, such as alginate, and techniques to develop scaffolds with promising mechanical properties. Figure 2 illustrates the use of alginate in corneal repair—drug delivery and cell delivery-based approaches.
Figure 2. Alginate in corneal repair—drug delivery and cell delivery-based approaches illustrated.
Download figure:
Standard image High-resolution imageCollagen-based approaches as well as other synthetic and natural biomaterials have been thoroughly reviewed by Matthyssen et al (2018) and will not be discussed here.
4.1. Alginate as a drug delivery system for the eye surface
Although the eye is the most accessible organ, ocular drug delivery is challenging because of low drug absorption when conventional liquid topical treatment is administered. Around 5% of the delivered drug penetrates the cornea and reaches the intraocular space. Lacrimal secretion, nasolacrimal drainage, and difficulty in penetrating the corneal epithelium are the primary causes of significant drug loss in the first 15–30 s post-administration, making it necessary to frequently repeat the application of concentrated solutions to achieve the desired therapeutic effect. Bandages or soft lenses equilibrated with drugs have been examined as potential drug carriers, however, have been found inefficient at sustaining prolonged drug delivery, as after 1–2 h these carriers become completely devoid of the drug (Ludwig 2005, Barbu et al 2006). Numerous other ophthalmic vehicles such as inserts, ointments, and suspensions have been developed to improve bioaccessibility; however, their side effects, such as blurred vision and necessity of repeated administration, result in low patient compliance. The perfect ophthalmic formulation would be in eyedrop form that does not cause blurred vision or irritation. An ideal ocular drug carrier should be non-cytotoxic, biocompatible, and biodegradable, in addition to having a loading capacity that ensures therapeutic benefit (Lin and Sung 2000). Site-specific delivery, continuous drug release, and increased precorneal residence should also be provided, enhancing drug permeability, prolonging the drug–cornea contact, and thus improving ocular drug absorption. Therefore, viscosity-enhancing agents or mucoadhesive polymers are ideal for overcoming drug absorption inefficacy (Khare et al 2014). Alginate possesses the desired characteristics due to its mucoadhesiveness. The high mucoadhesive strength of alginate is due to the free carboxyl groups in its backbone, which when ionized form hydrogen and electrostatic bonds, allowing alginate to interact with mucin. However, environmental pH and attenuation of alginate functional groups due to excessive hydration of the alginate polymer matrix in physiological fluids can impair this process (Taylor et al 2005, Mythri et al 2011, Haugstad et al 2015, Szekalska et al 2016). Alginate can be used alone or in combination with other biomaterials to improve drug delivery for ocular therapy (Lin et al 2004, Liu et al 2006). The ocular residence time increases with prolonged release of active agents from alginate microparticles due to alginate's mucoadhesiveness (Khare et al 2014).
Lin et al (2004) produced a range of pilocarpine-loaded alginate–pluronic hydrogels with the purpose to act as in situ gelling vehicles for delivery of pilocarpine to the ocular surface. Pilocarpine is used in eye drops for a variety of conditions, such as ocular hypertension and angle-closure glaucoma prior to surgery, and also to induce pupil constriction after dilation. The authors have determined the rheological parameters of alginate, pluronic and alginate-pluronic solutions and have suggested that the optimum concentrations to induce in situ gelation were 2% (w/w) alginate solution, 14% (w/w) pluronic solution and 0.1% alginate with 14% pluronic composite solution. The latter displayed significant increase in gel strength in physiological conditions as well as free flowing ability under non-physiological conditions (pH 4.0 and 25 °C). Results from pharmacological studies, conducted both in vivo and in vitro, indicated that by combining the two polymer systems improved retention of pilocarpine can be achieved when compared to retention in individual solutions. The authors suggest that this composite solution can be administered to the patient's eye in the form of ocular eye-drops forming a strong gel following phase transition and will be able to withstand the shear forces in the cul-de-sac. Based on these key findings the authors propose that alginate–pluronic composite hydrogel formulation showed enhanced bioavailability of pilocarpine compared to alginate or pluronic alone and that alginate–pluronic composite solution can be repeatedly administered to the eye surface without cytotoxicity, thus has therapeutic potential (Lin et al 2004).
Similarly, Liu et al (2006) reported improved efficacy when an alginate–hydroxypropyl methylcellulose (HPMC) composite hydrogel was used to encapsulate the antibacterial agent gatifloxacin, which is routinely used to treat external infections of the eye, such as acute and subacute conjunctivitis, bacterial keratitis, and keratoconjunctivitis. The drug delivery system was based on the principles of ion-activated in situ gelation, with alginate as a gelling agent and HPMC as a viscosity-enhancing agent. The authors report no change in rheological behaviors in all the tested composite alginate-HPMC solutions with the addition of gatifloxacin. Both in vitro and in vivo pre-corneal retention studies indicated that addition of alginate to the HPMC demonstrated improved gatifloxacin retention when compared to alginate or HPMC solutions alone. In the in vivo study, the composite gel demonstrated sustained drug release over 8 h, extending gatifloxacin's precorneal residence time. These key findings suggest that alginate-HPMC mixture can be used successfully as an in situ gelling vehicle to enhance drug bioavailability at the corneal surface. The authors hypothesize that the ease of administration, compared to inserts or contact lenses, could aid with patient acceptance and compliance (Liu et al 2006). Both studies conclude that in situ–formed composite alginate hydrogels demonstrate improved drug retention and enhanced ocular bioavailability without cytotoxicity and are viable therapeutic options to enhance drug delivery to the surface of the eye (Lin et al 2004, Liu et al 2006). Tokuda et al (2012) investigated the effects of a carteolol-containing alginate-based ophthalmic formulation on corneal epithelial barrier function in an in vivo clinical trial of ten healthy participants. To assess corneal epithelial barrier function an index was created based on the fluorescein uptake measured using Kowa FL-500. Additionally, to evaluate tear dynamics a Schirmer test was conducted. Carteolol is a nonselective beta-blocker routinely used to treat glaucoma. It inhibits the production of anti-inflammatory factors tumor necrosis factor alpha (TNF-α) and interleukin (IL)-6. The findings of this study suggest that presence of alginic acid does not affect corneal epithelial barrier function and possesses a water retentive effect. The authors report a long-acting retention of carteolol on the ocular surface in the group that received the alginate containing treatment. Alginate also lowered the IOP due to its ability to retain water. The authors suggest further investigation over longer periods of time, as the results reported in this study were obtained in a very short installation period of 7 d. However, based on the results the authors conclude that an IOP lowering effect was observed in the patients who received the 2% Mikelan Long-Acting Ophthalmic Solution that contained alginic acid and that alginic acid acted in a complementary manner to achieve this result (Tokuda et al 2012).
Another promising approach in the field of ocular drug delivery is using drug-loaded NPs. NPs effectively encapsulate drugs and can be designed for prolonged drug delivery to the ocular mucosa (Motwani et al 2008, Liu, Griffith and Li, 2008, Nagarwal et al 2012, Zhu et al 2012). Motwani et al (2008) loaded chitosan–alginate NPs with gatifloxacin to prolong the contact time of the antibiotic with the ocular surface. Chitosan is a cationic polysaccharide and thus can interact with anionic alginate readily. Chitosan is widely used in ophthalmic therapy because of its biocompatibility, non-cytotoxicity, and ability to enhance drug penetration across the mucosal epithelium (Alonso and Sánchez 2003, de La Fuente et al 2010). In this study, a modified coacervation or ionotropic gelation method was used to produce submicroscopic nanoreservoir systems. Atomic force microscopy, FT-IR, DSC and TEM characterization was performed on the NPs, elucidating drug particle size, content, encapsulation efficiency, polydispersity index and zetapotential. The formulation composed of 0.22% chitosan, 0.38% alginate and 0.05% gatifloxacin was discovered as an optimum formulation. In this optimum formulation the encapsulation efficiency was 79.63% with particle size and zetapotential of 347 nm and +38.6 mV, respectively. The burst release from the formulation was 11.08%. In vitro release studies demonstrated a fast release during the first hour followed by a more gradual, sustained release of gatifloxacin over subsequent 24 h following a non-Fickian diffusion process. These findings support that alginate-chitosan composite gels are able to be a new vehicle for prolonged topical ophthalmic delivery of antibiotics in a therapeutic setting and can be a viable alternative to eye drops for its ease of administration, reduced dosing frequency and can result in better patient compliance (Motwani et al 2007). Liu, Griffith and Li, (2008) incorporated bovine serum albumin (BSA) in alginate microparticles in a composite collagen hydrogel developed for ocular applications. The purpose of BSA was to serve as a model drug. The authors report that the composite collagen hydrogel with encapsulated alginate microparticles remained optically transparent and retained greater mechanical robustness when compared to control hydrogels without microspheres. The initial loading efficiency of BSA of ∼63.6% was obtained. The authors report sustained release of the drug over an 11 d period in in vitro conditions. The composite hydrogel was successfully able to maintain human epithelial cell growth and proliferation. These findings have led the authors to suggest using their composite hydrogel as a therapeutic lens for sustained drug delivery or even as a potential corneal alternative to be transplanted into patients with corneal diseases. Composite hydrogels with the addition of natural materials, such as alginate, are therefore useful in future therapeutic applications as bio-interactive implants, inserts or contact lenses (Griffith et al 2008).
To improve drug bioavailability in conjuctival corneal squamous cell carcinoma, Nagarwal et al (2012) encapsulated 5-fluorouracil (5-FU) in chitosan-coated sodium alginate–chitosan NPs for ophthalmic delivery. 5-FU is a pyrimidine analog commonly used to treat many epithelial cancers. Ionic gelation technique was employed to produce the drug-laden alginate-chitosan NPs, which subsequently were suspended in chitosan solution. The size and morphology of the NPs was characterized by dynamic light scattering, scanning electron microscopy, atomic force microscopy and zeta potential. Dialysis membrane technique was used to study drug release in vitro. The authors report that both the size of the NPs and the drug encapsulation efficacy depended on molar ratio of alginate and chitosan used. Additionally, the size of NPs further increased after coating with chitosan. The authors have not observed any interaction with mucin in their in vitro studies with systems tested, however do report an increase in viscosity of NPs after chitosan coating and sustained drug release with high burst effect. In the in vivo studies performed with rabbits, greater levels of 5-FU in the aqueous humor were observed in eyes treated with the composite system, compared to the control. Optimized formulation has shown no irritation as tested by the modified Draize test. Thus, the authors conclude that the chitosan coated alginate-chitosan NPs are effective as a topical application for ocular drug delivery (Nagarwal et al 2012). However, chitosan has poor water solubility at a pH of <6, which can cause aggregation and precipitation, limiting the efficacy of the system at absorption sites at neutral pH (Zhu et al 2012). Zhu et al (2012) attempted to overcome this limitation and further improve mucoadhesiveness and ocular drug delivery by using thiolated chitosan–sodium alginate and reported that this formulation is more stable and effective than chitosan–sodium alginate NPs alone. They hypothesized that thiolated polymers improve mucoadhesive properties by forming disulfide bonds between thiol groups and mucus glycoproteins. The authors characterized their preparation method with particle size, zeta potential, SEM and mucoadhesion studies. The results show that thiolated system demonstrates smaller size (265.7 ± 7.4–471.0 ± 6.4 nm), higher positive charges (49.2 ± 2.3–29.5 ± 4.1 mV) and higher mucoadhesion properties. The amount of thiol immobilized was tested with Ellman's reagent. The method demonstrated a high degree of thiol substitution, up to 1.411.01 ± 4.02 μmol g−1. In vitro cytotoxicity studies using human corneal epithelial cells showed no cytotoxic effects (almost 94% cell viability as determined by MTT assay). In vivo studies were performed using male Wistar rats and demonstrated greater drug bioavailability compared to alginate-chitosan NPs alone, indicating that thiol substitution has a beneficial effect for creating improved methods of ocular drug delivery (Zhu et al 2012).
Moore et al (2013) used an in vivo (rat) corneal wound model to assess a newly designed peptide capable of promotion of wound healing and epithelial regeneration. The authors show that alpha-carboxy terminus 1 peptide (αCT1) from the C-terminus of connexin 43 can be encapsulated in polymeric alginate–poly-l-ornithine (A-PLO) microcapsules. αCT1 has been successful at decreasing healing time and reducing the degree of scar tissue formation in porcine excisional skin wound models. In this study, the authors compare the efficacy of delivery of αCT1. The peptide was delivered directly, in combination with a concentrated pluronic solution or using a sustained release A-PLO system. Analysis of wound healing was performed using histology and fluorescent microcopy and a significant reduction in regeneration time has been observed in αCT1 microcapsule treated rat corneas compared with controls (88% vs 38%). A reduction in inflammatory response was also evident in corneas treated with αCT1 microcapsules when compared to the pluronic system. These key findings demonstrate that αCT1 encapsulated in A-PLO produced at least 50% improvement in the corneal wound-healing time compared to the control, demonstrated sustained release with minimal cell loss and reduced inflammation, suggesting this treatment's benefits in corneal wound healing (Moore et al 2013).
Noreen et al (2020) tested the therapeutic effect of their pH triggered in situ gelling system containing moxifloxacin (MFX) hydrochloride (MOX-HCl), sodium alginate and natural gum derived from Terminalia arjuna bark exudate as a gelling agent. MOX-HCl is a fourth generation 8-methoxyfluoroquinolone broad spectrum antibiotic designed specifically for ocular applications, as it has previously demonstrated enhanced potency compared to other topical antibiotics. Dol et al (2014) have demonstrated the effective dose of MOX-HCl to be the administration of one drop of the solution twice a day for a week (Dol et al 2014, Noreen et al 2020). The tested ophthalmic formulation was transparent in nature, had the potential to form a gel when in contact with tear fluid and was examined by performing the disc diffusion method assay to test anti-microbial activity against four bacterial strains (Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Salmonella typhae) and two fungal strains (Trichoderma and Fasorum oxisporum). High antibacterial activity of the formulations was observed compared to the control. In terms of antifungal activity, the formulations exhibited no zones of inhibition, however also no fungal development on the surface of the strips has been detected. Cytotoxicity of the formulation was assessed by performing metabolic analysis. Negligible cytotoxic effects were reported, and the authors suggest that the formulation is safe to use in the human eye. Additionally, ex vivo ocular irritation studies, ex vivo transcorneal permeation studies and in vivo modified Draize eye irritation test were performed to assess the safety and efficacy of the developed formulation with promising outcomes. Overall, the therapeutic formulation was stable, therapeutically efficacious, non-irritant, successfully converted from sol to gel in the cul-de-sac of the eye during the in vivo studies with rabbits and sustained release of the drug (over 12 h) was reported (Noreen et al 2020). Chang et al 2020 have also developed a composite drug releasing ophthalmic formulation comprising of chloramphenicol-loaded liposomal NPs, 200 nm in diameter, that was called LipoCAP, combined with a mixture of collagen, gelatin and alginate gel that was termed (CGA). Chloramphenicol is an amphenicol antibiotic routinely administered to the surface of the eye to treat a variety of bacterial infections including conjunctivitis. The degradation rate of the formulation was assessed and determined to be 8 h with the formulation reaching the effective working concentration in 75 min and the overall drug release time was sustained for at least 12 h. The formulation was able to inhibit proliferation of E. coli consistently with inhibition being more prominent over time. Epithelial cells were co-cultured with the novel formulation with no observed significant toxicities, no in vivo tests were performed. The authors conclude that their novel formulation accomplished effective chloramphenicol dose concentration in a short time as well as achieved sustained delivery of chloramphenicol over time, suggesting it would be a useful therapeutic ophthalmic formulation able to avoid pre-corneal drug loss (Chang et al 2020). The authors have also published their research findings using the same mode of delivery yet with MFX and dexamethasone (DEX)-loaded nanostructured lipid carriers that they termed (Lipo-MFX/DEX). The same mixture of CGA was used to improve pre-corneal residence of the compounds. MFX is a routine antibiotic used for the treatment of a range of bacterial infections and DEX is a synthetic glucocorticoid with anti-inflammatory and immunosuppressant properties used to alleviate eye pain and discomfort following eye surgery. The authors have reported drug entrapment efficiency of 91.5 ± 3.5% and observed the delivery of the effective working concentration of the drugs in 1 h with sustained release for at least 12 h. When the formulation was tested in a co-culture system with corneal epithelial cells no cytotoxicity was observed in all the tested conditions. Interestingly, the authors reported increased cell survival rates in the CGA-Lipo-MFX/DEX group (216.2 ± 20.7%) compared to the CGA-Lipo-MFX group (91.5 ± 3.5%) and the CGA-Lipo-DEX group (186.7 ± 18.1%). The authors hypothesize that the addition of DEX into the system has promoted epithelial cell proliferation and suggest that this property might aid in corneal epithelial wound healing. Further in vitro testing of the therapeutic compound was performed on enucleated mouse eyes to quantify the wound healing effect. The mice have undergone epithelial debridement in the centre of the cornea, sacrificed, the eyeballs were isolated and cultured with topical administration of exogenous CGA alone, CGA-Lipo-MFX, and CGA-Lipo-MFX/DEX. The authors report turbidity in the eyes that received CGA treatment alone at day one and no turbidity in the eyes treated with CGA-Lipo-MFX and CGA-Lipo-MFX/DEX up until day 3. Later time points are not discussed in the paper. Additionally, the levels of leukocyte or lymphocyte infiltration were assessed by performing haematoxylin and eosin staining on the enucleated eyes that have been incubated in the co-culture system with the tested conditions. In the CGA only group, increased levels of inflammation and oedema progression were observed without epithelial regeneration as observed on day 3. In contrast the CGA-Lipo-MFX group and the CGA-Lipo-MFX/DEX group showed significantly lower degrees of oedema and dramatically reduced number of infiltrating immune cells with improved epithelial cell restoration. This evidence suggests that the novel formulation containing MFX and DEX can support antimicrobial activity as well as reduce inflammation, enhance corneal epithelial cell proliferation thus accelerating corneal wound healing (Chang et al 2020).
Another important parameter that should be considered is the degradation rate of alginate. Depending on its concentration, the degradation rate of alginate can be slow and uncontrollable; in addition, the release of high-molecular-weight strands might cause complications with clearance from the host's body (Bouhadir et al 2001). Bouhadir et al (2001) proposed a possible solution to this problem. In their study, commercially available high-molecular-weight alginate was oxidized with sodium periodate to cleave the carbon–carbon bond of the cis‐diol group in the uronate residue, creating acetal groups susceptible to hydrolysis and thus altering the confirmation of the molecular backbone in order to promote more rapid hydrolysis of alginate in an aqueous environment (Boontheekul et al 2005). Oxidized alginate was used to prepare gels by cross-linking with calcium ions, degradation behavior was assessed by measuring the elastic modulus and weigh loss over time. Additionally, size-exclusion chromatography was performed over time as a function of pH or temperature of the solution to test the degradation of oxidized alginate. The authors concluded that degradation of oxidized alginate depended on the pH and temperature of the solution, and complete degradation of hydrogels was achieved in 9 d in PBS solution (pH 7.4, 37 °C). Boontheekul et al (2005) also characterized the degradation rate of either alginate gels oxidized with sodium periodate or high-molecular-weight alginates gamma-irradiated with cobalt-60, as previously described (Kong et al 2002, Boontheekul et al 2005). Analogously, degradation was due to hydrolytic chain scission, and the rate of degradation depended on the degree of oxidation and the molecular weight of alginate, as analyzed by the reduction of mechanical properties and corresponding number of crosslinks, dry weight loss, and molecular weight decrease. The cytocompatibility of the modified scaffolds was then assessed by culturing C2C12 myoblasts on top of them. The cells were able to adhere, proliferate and differentiate on the modified gels just as well as on the control gels, indicating that these hydrogels show a degree of tunability and are powerful tools for use in TE (Boontheekul et al 2005). This tunability of alginate gel degradation renders the material powerful for TE and future use in transplantation.
All the studies mentioned above demonstrate that considerable scientific interest in the last 20 years in utilizing alginate based approached for improving drug delivery to the ocular surface is maintained. Novel combinations of biomaterials encapsulating a variety of therapeutic agents keep emerging with promising results.
In conclusion, polymeric materials exhibit good drug bioavailability and have great potential in the clinical setting. Provision of bioactive molecules in a controlled fashion continues to be an essential component of engineering strategies in tissue defect repair.
4.2. Alginate in corneal repair: cell delivery–based approach
TE involves the combination of cells and or signaling molecules such as proteins and growth factors with appropriately designed scaffold materials that are then co-delivered to a native injured area to encourage cellular/tissue regeneration. As mentioned in the previous section, drug delivery schemes can range from introduction of simple drug-laden microparticles to the affected area to use of more complicated composite systems where drugs are immobilized by covalently linking to the scaffold (Ekenseair et al 2013). Additionally, the incorporation of tunable drug delivery mechanisms to the tissue engineered scaffolds would further aid in stimulating tissue regeneration or formation. Drug release kinetics can be adapted by including a coating (e.g. heparin) that binds cells or drugs in a specific or non-specific manner, depending on the application (Rambhia and Ma 2015). Cell delivery strategies to the target site replace lost or damaged tissue either through direct integration or by producing a therapeutic effect via paracrine mechanisms, such as the release of hormones, growth factors, or cytokines by the delivered cells to indirectly alleviate the severity of the damage. The versatility, tunability, and injectability of alginate hydrogels, as well as their ECM-like features, renders them efficient for cell delivery for tissue regeneration.
The morphology of corneal keratocytes encapsulated in alginate hydrogels remains spherical. The cells do not exhibit spreading behavior with no evidence of cellular protrusions in non-functionalized alginate gels. Encapsulated corneal keratocytes are viable for at least 7 d in standard tissue culture conditions and are immobilized in non-functionalized alginate, however, have been shown to migrate within low percentage RGD functionalized alginate (Neves et al 2020). These observations are consistent with research conducted on mesenchymal stromal cells (MSC). MSCs have been demonstrated on multiple occasions to not interact with non-functionalized alginate as is the case with most anchorage dependent cells (Swioklo and Connon 2016, Connon et al 2020). Multiple tissues and organs have been regenerated using alginate-based cell delivery systems (Bidarra et al 2014). However, there are few studies using alginate as a cell delivery system for corneal regeneration in vivo. Liang et al (2011) produced an oxidized, in situ–formed alginate dialdehyde-based hydrogel with hydroxypropyl chitosan to facilitate corneal endothelium reconstruction. Rabbit corneal endothelial cells encapsulated in the composite hydrogel had the capacity to grow on Descemet's membrane in vivo, with a minor inflammatory response. In addition, the self-crosslinking nature of the gel reduced cytotoxicity and improved the in vivo degradation rate. Overall, the transplanted endothelial cells connected to the damaged Descemet's membrane of the host and successfully alleviated post-operative corneal opacity over 120 d compared to the negative control group, where the cornea remained thick and opaque (Liang et al 2011). Xu et al (2018) used an in situ self-crosslinking alginate–chitosan hydrogel to transplant primary rabbit limbal SCs to facilitate corneal epithelium reconstruction in a rabbit alkali burn model. There was a mild inflammatory response immediately post-injection, which decreased over time. The transplanted limbal SCs supplemented the injured epithelial cells in the wounded tissue and had a rapid restorative effect on corneal integrity compared to untreated negative controls. This remedial effect was probably due to the combination of a hydrogel and the paracrine effects of limbal SCs. Chitosan reduces fibrosis, thus inhibiting scar tissue formation promoted by over-activated corneal keratocytes as a response to injury (Li et al 2016, Ren et al 2016). Encapsulated limbal SCs maintain their viability and even display a certain degree of proliferation and differentiation. In addition, high hydrogel transparency, high swelling capacity, high cytocompatibility with limbal SCs, biocompatibility with the host tissue, and biodegradability are observed (Xu et al 2018).
Connon et al (2020) demonstrated the importance of the paracrine healing effect in corneal wound healing. They investigated the therapeutic potential of human adipose-derived mesenchymal stromal cells (Ad-MSCs) encapsulated in alginate gel bandages in an in vitro scratch assay and an in vivo mouse model. The alginate bandages maintained the viability of the Ad-MSCs at room temperature for 3 d prior to being applied to adult mice corneas subjected to epithelial debridement. The treatment group showed decreased inflammatory cell infiltration compared to the control group in vivo, probably due to the release of soluble anti-inflammatory factors produced by the Ad-MSCs. In vitro studies confirmed the presence of anti-inflammatory factors, such as tumor necrosis factor–inducible gene 6, hepatocyte growth factor, IL-8, and monocyte chemoattractant protein-1. Moreover, alginate-encapsulated Ad-MSCs maintained their specific SC marker expression and produced beneficial outcomes, such as corneal haze improvement (Connon et al 2020).
Alginate has been used as a cell carrier for corneal injury treatment in vivo. The use of alginate as a scaffold material in vitro for future in vivo studies will be discussed next.
4.3. Alginate as a support structure: a scaffold
An ideal biomaterial for successful corneal TE should have a suitable refractive power, the potential to provide optimal conditions for corneal stromal fibroblasts, sufficient rigidity, and compatibility with the tension induced by high IOP and eye movements (Ruberti and Zieske 2008, Tonsomboon and Oyen 2013). Alginate hydrogels are often used as a support structure for corneal TE, because of the similarity to the native tissue in terms of water content (80%). However, alginate hydrogels do not have the same highly organized fibrous structure as the native ECM, thus showing limited stability and rigidity. In addition, the fibrous structure acts as a spatial guide for corneal fibroblasts for their organized ECM secretion in vivo (Wu et al 2014). To overcome this issue, multiple studies have attempted to modify alginate-based scaffolds. Strange et al (2014) produced composite alginate hydrogels incorporated with electrospun polycaprolactone. Although the mechanical properties of the hydrogels improved significantly and the authors reported the study provides a way to create large-scale biomimetic constructs that are able to mimic native tissue with tunable and compressive properties, the constructs lost their suitability for corneal TE applications due to opacity (Strange et al 2014).
Building on this research, Tonsomboon and Oyen (2013) reinforced alginate hydrogels with electrospun gelatin nanofibers. The authors used gelatin because it is cheaper than native collagen and also is electrospinnable and nontoxic. There was a significant increase in the tensile elastic modulus of the reinforced hydrogels from 78 ± 19 to 450 ± 100 kPa, which brought the scaffold closer to the stiffness of the native cornea (0.579–4.9 MPa) (Elsheikh et al 2008, Ruberti and Zieske 2008, Knox Cartwright, Tyrer and Marshall, 2011). However, during gelatin fiber–carbodiimide hydrochloride crosslinking, which is essential for preventing rapid scaffold degradation in vivo post-transplantation, the hydrogels lost their transparency again, rendering them unsuitable for corneal scaffold engineering. Therefore, no studies determining corneal cell viability in the scaffold were performed (Tonsomboon and Oyen 2013).
Wright et al (2013) investigated the compatibility of another alginate-based scaffold with corneal epithelial cells. Prior to encapsulation of the cells in the scaffold, alginate was oxidized. Larger internal pores were observed in the oxidized alginate and corresponding lower stiffness of the material compared to the unmodified control. There was no significant difference in the viability or differentiation capacity of corneal epithelial cells in the oxidized and control alginates. The encapsulated corneal epithelial cells retained their capacity to secrete proteins rich in cysteine, profilin-1, and galectin-1 into the media, producing conditioned media, which have an inhibitory effect on corneal re-epithelialization (Reakasame and Boccaccini 2018). The authors hypothesized that adding type IV collagen to the oxidized alginate would lead to higher cell viability compared to the control alginate (Wright et al 2013).
With the emergence of 3D bioprinting technology, alginate has been extensively used in bioink development. The main objective of 3D bioprinting is to create a 3D scaffold from biomaterials and living cells. The advancements in using alginate in bioink development will not be reviewed here (Datta et al 2020). Isaacson et al (2018) incorporated sodium alginate with methacrylated type I collagen in a bioink and successfully fabricated corneal structures resembling the native cornea structure. Corneal keratocytes were encapsulated in the bioink and exhibited high cell viability on both the day of printing (>90%) and 7 d later (83%). The alginate–collagen composite bioink had sufficient stiffness to support the corneal curvature, which was also successfully maintained after detachment from the bioprinting support bath. These findings show that 3D bioprinting is a feasible technique for creating artificial corneas (Isaacson et al 2018). Above mentioned studies have been summarized in terms of method of preparation of the composite scaffolds and the advantages and disadvantages of the study in supplementary table 1 (available online at stacks.iop.org/BMM/17/022004/mmedia).
4.4. Alginate for cell storage and transport
Alginate-based hydrogels can be used to encapsulate and immobilize different types of cells (Krol et al 2006, Kintzios et al 2007, Wikström et al 2008, Cabané et al 2009, Hunt et al 2009, Cook et al 2011). Alginate is bioinert and semipermeable, making it an attractive biomaterial suitable for storage and transport of transplantable cells. Alginate-based hydrogels can be used for short-term storage and transport of corneal epithelial cells to be used for the reconstruction of a functional corneal epithelium in LSCD (depletion of epithelial cells in the limbal region of the cornea) patients (Wright et al 2012). Currently, corneal epithelial cell transplantation is the main therapy performed in a few specialist hospitals in developed countries. The cells for transplantation are expanded in vitro on amniotic membranes in discrete laboratory facilities associated with the hospitals where the procedure takes place. Therefore, the ability to store and transport these cells in alginate opens up the possibility of setting up distribution centers to increase the availability of this therapy for patients who live further away from the hospitals. Due to the ability to rapidly and easily retrieve alginate-encapsulated cells when needed, Wright et al's (2012) method has clear advantages compared to conventional corneal tissue or cell storage. Damala et al (2019) encapsulated human limbus–derived stromal/mesenchymal stem cells (hLMSCs) in alginate beads to facilitate cell transport from bench to bedside for corneal scar treatment. The cells were encapsulated for 3–5 d at room temperature or 4 °C and then released and returned to culture. The cell viability post-release from room temperature storage in alginate was >80% after 3 d storage and >75% after 5 d storage, higher than the cell viability after storage at 4 °C. In addition, the cells stored at room temperature had a higher survival rate and maintained their characteristic phenotype. The findings show that alginate encapsulation is an effective method of preserving hLMSCs and provides high cell viability over prolonged durations in transit at room temperature, potentially expanding the scope of cell-based therapy for corneal blindness (Damala et al 2019).
5. Obstacles to overcome in corneal tissue engineering and future perspectives
Alginate based materials have shown potential to achieve the vision of effective, non-invasive corneal wound healing and regeneration. Alginate can be a useful tool for drug delivery to the ocular surface because of several advantages, such as ease of encapsulation of therapeutic agents, ease of administration (minimally invasive local delivery) compared to inserts and contact lenses, which can improve patient adherence and compliance, mucoadhesive characteristics that enhance sustained drug release subsequently improving corneal repair and regeneration, biocompatibility and lack of immunogenicity, the presence of alginic acid does not affect corneal epithelial barrier function in vivo and has been shown to lower IOP, however, a number of obstacles remain to be overcome.
One of the biggest challenges in corneal TE is engineering the corneal stroma, due to the difficulty in reproducing the highly ordered structure of the tissue, with exceptional biomechanical properties which give rise to the optical transparency. In standard cell culture conditions, corneal stromal cells can lose their phenotype and adopt fibroblastic phenotype when expanded in serum containing medium. This phenotype change leads to the secretion of disordered ECM by the fibroblasts similar to what can be found in opaque corneal scars. Keratocytes lose their ability to produce cornea-like ECM after multiple population doublings. However, recently, Gouveia et al (2019) have demonstrated that by regulating the topographical cues in vitro, corneal stromal fibroblasts can be persuaded to align in a native-like fashion (2020). Other engineering strategies for the corneal stroma include decellularize animal alternatives, however immune rejection remains a problem that still requires further research.
One of the biggest obstacles in corneal endothelial TE is the intrinsic limited regenerative capacity of endothelial cells, which constrains in vitro cell expansion and delays the development of therapeutic agents for devastating diseases such as Fuch's endothelial dystrophy, age-related endothelial cell density loss and corneal oedema. Transdifferentiation of endothelial cells to the mesenchymal phenotype during cell culture leads to the adoption of the fibroblast-like phenotype by the endothelial cells, with loss of hexagonal, cobble-stone morphology and loss of cell-to-cell contact. It has been noted by many researchers in the field (Leonard et al 2016, Zhao et al 2019, Tsai and Daniels 2021) that biomechanical changes to the corneal ECM, such as corneal curvature, stiffness, transparency, and regularity are associated with corneal disease progression. The relationship between the dynamic changes in the biomechanics of the cornea and disease needs to be elucidated comprehensively in order to develop successful therapeutic strategies. For example, Leonard et al 2016 have discovered that stiffness changes in the Descemet's membrane paves the way for subsequent endothelial morphological changes and cell density loss. Accumulation of atypical ECM structures on the Descemet's membrane alter biomechanical properties of the tissue and affect ECM remodelling and cell proliferation (Leonard et al 2016, Tsai and Daniels 2021). This interaction of endothelial cells with their surrounding ECM is termed the signalling process of mechnotransduction. By understanding mechnotrasnduction and the biochemical properties of native ECM, improved tissue engineered scaffolds that support endothelial cell proliferation and maintain cell morphology and function can be formulated. The use of natural materials such as collagen type I, chitosan or gelatin to create suitable substrates have been suggested to provide adherence motifs and encourage cell-matrix interaction as well as simulate native tissue in terms of porosity and geometrical features (Liang et al 2011, Tsai and Daniels 2021). Another barrier to overcome in devising successful treatment strategies for endothelial cell damage and maintenance of healthy homeostasis and transparency in the cornea is IOP, which under normal circumstances is regulated by corneal endothelial cells providing a barrier to divide the corneal stroma from the aqueous humour. Atypical resistance to the drainage of aqueous humour can build up IOP, which can lead to endothelial cell loss and corneal oedema due to disruption of the integrity of endothelial tight junctions and the sodium-potassium ATPase pump functions.
Despite the advancements achieved in in vitro studies, few clinical trials have been performed on humans. Further to this, approaches for drug delivery can only treat a certain range of disorders to a degree of severity. TE poses a potential strategy for addressing the growing need of corneal transplants. Great strides have been made with the approval of Holoclar and other corneal epithelial replacements, yet these are non-applicable in cases of advanced deep corneal pathologies including stromal melt and endothelial damage.
Based on the recent literature, the future use of alginate in corneal TE will encompass creation of hybrid scaffolds or ophthalmic formulations. We envisage that alginate based therapeutic solutions (either NPs or hydrogels that encapsulate therapeutic agents) will be further developed and utilized as drug delivery vehicles by fine tuning degradation, improving pre-corneal residence of therapeutics and drug release rates of the growth factors, antibiotics or other medicinal agents used, depending on the exact application or condition being treated.
6. Conclusion
Improvements in therapeutic strategies for corneal disease treatments have enabled to move from corneal replacement to corneal wound healing and regeneration. The adaptability and versatility of alginate hydrogels, in addition to their ECM-like features, are the key factors for using them as biomaterials in corneal TE and regeneration. By modulating its biophysical properties, alginate can be fine-tuned to suit therapeutic strategies for treating corneal diseases. Alginate is compatible with both drug and cell delivery systems. Using alginate as an injectable material has several advantages, such as easy incorporation of therapeutic agents and minimally invasive local delivery, enhancing regeneration are multiple approaches using alginate, each with its benefits and limitations. However, one major drawback is the inability to predict in vivo outcomes on the basis of in vitro studies. Advancements in 3D microfabrication technologies using alginate-based bioinks have shown promise in creating a corneal stromal equivalent. Corneal stroma and endothelium TE has made noticeable progress; however, a truly biomimetic, whole-thickness corneal alternative comprising native cell types and orthogonally arranged lamellae has not yet been produced. Future studies need to combine biomimetic strategies, such as cell signaling agents, with tissue-engineered scaffolds to promote native ECM deposition and natural tissue organization for restoration of functionality.
Acknowledgments
The authors acknowledge financial support from the Intensive Industrial Innovation Programme, part of the European Regional Development Fund. We wish to extend a special thank you to Dr. Dean Hallam for helping with the figures in this paper. We also wish to show our appreciation to the anonymous referees for their helpful suggestions.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).