Abstract
Background It was hypothesized that radiofrequency denervation (RFD) of lumbar facet joints is associated with superior pain abolishment and less complications than chemical neurolysis (with ethyl alcohol or glycerol) in patients with chronic facet joint arthropathy.
Methods For this prospective cohort study, adult patients with chronic lumbar facet joint arthropathy were prospectively enrolled between 2017 and 2019. The following groups were compared before the intervention and 6 weeks, 6 months, and 12 months after the intervention: RFD, chemical neurolysis with ethyl alcohol 95% (EA-95), or glycerol 20% (Gly-20). Outcome parameters included the Core Outcome Measures Index for the back (COMI-back), World Health Organization (WHO) pain ladder level, and visual analog scale (VAS). P values <0.05 were considered statistically significant.
Results A total of 95 patients with a mean age of 63.7 years were included. Among them, 30 patients underwent RFD, 30 patients were treated with EA-95, and 35 individuals were treated with Gly-20. After 6 weeks, RFD patients had significantly lower VAS scores compared with the EA-95 group. After 6 months, both VAS and COMI were significantly lower in RFD patients than in the Gly-20 group. Twelve months after intervention, VAS scores were significantly lower in the RFD group compared with the Gly-20 group.
Conclusions This study reveals that RFD is associated with improved pain relief and quality of life compared with chemical neurolysis for facet joint-related chronic lower back pain and should be considered as the treatment of choice in patients with chronic low back pain due to facet joint arthropathy.
Clinical Relevance The current study provides information that may improve clinical decision making in the treatment of chronic lumbar facet joint arthropathy and to appropriately counsel such patients about expected outcomes.
Introduction
Chronic low back pain is considered a major public health problem worldwide.1 Nontraumatic low back pain is associated with high disability rates and the inability to work. In Germany, the annual prevalence rate of chronic low back pain has been found to be as high as 75%.2 While the pathophysiology of chronic low back pain is multifactorial, it has been demonstrated that lumbar facet joint (zygapophysial)-related pain is involved in 15%–45% of cases.3 Lumbar facet joint innervation is orchestrated by the medial branches of the dorsal ramus of the spinal nerve, and it was previously described in detail by Bogduk and Long.4 Neurophysiological investigations of facet joint capsules have identified low-threshold mechanoreceptors and sensitive nociceptors. Local inflammatory processes may further lower these activation thresholds and increase baseline nerve discharge rates. Given the high level of strain on facet joints, as demonstrated in biomechanical analyses, it is tempting to hypothesize that this interplay makes facet joints prone to being significant contributors to chronic low back pain.5 Various minimally invasive treatment modalities for the management of recurrent lumbar facet joint pain have been implemented. Intra-articular lumbar facet joint injection, medial branch blockage therapy with steroids and/or local anesthetics, radiofrequency denervation (RFD), cryotherapy, and chemical neurolysis with ethyl alcohol (50%–100%), phenol (5%–10%), or glycerol (20%–100%) are being utilized.6–8
While intra-articular steroid injection therapy and medial branch blockage with steroids lead to effective transient pain relief, the long-term outcomes are suboptimal. Additionally, the role of chemical neurolysis for longer-term treatment of low back pain using different concentrations of ethyl alcohol, phenol, or glycerol is controversial. In 2010, the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine recommended that chemical denervation should not be used in the routine care of noncancer patients with chronic pain.9 There is moderate-to-strong evidence that RFD provides both short-term and longer-term relief of low back pain of facet joint origin.10 Comparative studies for medium-term and long-term treatment of chronic facet joint arthropathy are scarce, and neither success rates nor complications are known.6,11 Therefore, the aim of the current study is to determine both the short-term and medium-term clinical outcomes of different types of neurolysis. We hypothesized that RFD is associated with improved short-term and medium-term outcomes and fewer complications than chemical neurolysis (with ethyl alcohol 95% [EA-95] or glycerol 20% [Gly-20]) in patients with chronic lumbar facet joint pain.
Materials and Methods
Study Design and Ethical Approval
A prospective cohort study was performed in the department of spine surgery at our institution, an accredited level 1 spine center certified by the German Spine Society (DWG). Patients were enrolled in the study between December 1, 2017, and December 1, 2019. The protocol was approved by the regional ethics committee (file number: 2016448). Informed consent was obtained from all participants.
Cohorts
Adult patients with chronic low back pain resistant to noninvasive and steroid injection therapies were identified. Thereafter, patients were considered for inclusion if they met the specific criteria. The first criterion was a confirmed history of chronic, function-limiting low back pain of at least 6 months’ duration despite maximal conservative therapy, including oral pain medication, physiotherapy, and lifestyle optimization. The second was an absence of radicular symptoms. Furthermore, on physical examination, paraspinal tenderness should be present. The final criterion was increased pain upon hyperextension, rotation, or lateral bending of the lower lumbar spine.
All patients underwent routine magnetic resonance imaging of the lumbar spine to exclude alternative diagnoses and to confirm at least one of the following findings reflecting degenerative facet pathology:
Facet joint effusion
Facet osteophytes
Facet bone sclerosis
Facet joint narrowing
To localize the origin of lumbar back pain in more detail, all patients underwent diagnostic medial branch block therapy in which a mixture of 10 mL ropivacaine hydrochloride (20 mg/10 mL) (Ratiopharm GmbH, Ulm, Germany) and 1 mL triamcinolone acetonide (40 mg/1 mL) (Hexal AG, Holzkirchen, Germany) was injected into the lumbar facet joints L3/L4–L5/S1. If a short-term reduction of at least 50% in the visual analog scale (VAS) was seen, patients were considered candidates for facet neurolysis and subsequent inclusion in the study.
The following exclusion criteria were utilized: confirmed concurrent disc herniation, symptomatic radiculopathies, spinal instability, vertebral fractures, rheumatic disorders, neuromuscular disorders, history of opioid abuse, pregnancy, lactation, a history of adverse reactions to glycerol or ethyl alcohol, or written informed consent not obtained.
Execution of Diagnostic Medial Branch Blocks
All injections were performed in prone position and under intermittent fluoroscopic visualization (OEC Fluorostar, GE Healthcare, Chicago, IL, USA), with continuous monitoring of the patients’ vital signs (saturation and pulse rate) and frequent blood pressure measurements. X-ray imaging was conducted by an experienced technician.
Briefly, tender lumbar facet joints (L3/L4–L5/S1) were palpated, marked, and located with fluoroscopic guidance. Under aseptic conditions, a 22G needle was inserted until contact was made with bone at the edge of the facet joint. Correct needle positioning was confirmed with fluoroscopy. When the needle was in place, 0.5–1.5 mL of a mixture of 10 mL ropivacaine hydrochloride (20 mg/10 mL) and 1 mL of triamcinolone acetonide (40 mg/1 mL) was injected into the target joints. Eventually, the L3/L4, L4/L5, and L5/S1 facet joints were infiltrated bilaterally in all participants. Selective infiltrations of specific joints were not performed. Afterward, the injection site was disinfected again and covered with a plaster.
Enrollment and Grouping Procedure
After administration of the diagnostic medial branch block and the recurrence of lumbar facet joint pain, candidate patients were assessed to determine whether they met the inclusion or exclusion criteria.
After being provided with sufficient information regarding treatment options, all patients were given written informed consent documents, listing their actual diagnoses and an overview of the treatment options (including conservative options), including potential complications. The patients were offered 3 treatment options and were allowed to select the treatment modality they preferred. They were then treated and grouped according to that decision, as follows:
The Gly-20 group: chemical neurolysis with glycerol 20%
The EA-95 group: chemical neurolysis with ethyl alcohol 95%
The RFD group: radiofrequency denervation
Patients were free to obtain a second opinion or discuss the proposed treatment options with their general practitioners. After finalizing the decision-making process and obtaining informed consent, patients were scheduled to undergo the intervention and given a preintervention examination.
Execution of Radiofrequency Denervation
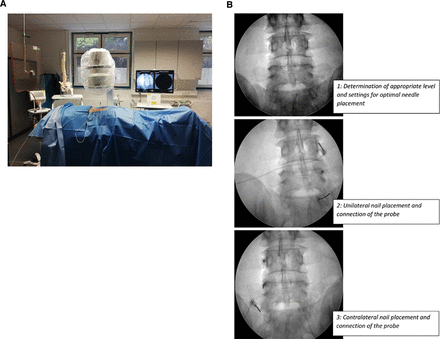
The participants were placed in prone position; all injections were performed under intermittent fluoroscopic visualization, and vital signs were continuously monitored. Figure 1A depicts the setup. Electrodes and disposable 22G curved radiofrequency needles with 100 mm active tips (NeuroTherm, Wilmington, MA, USA) were placed at the site of the medial branch of the dorsal ramus of the relevant L3/4–L5/S1 facet joints. A representative example of needle placement is shown in Figure 1B. Correct placement was confirmed using electrostimulation in the sensory testing (50 Hz, 0–1 V) and motor testing (2 Hz, 1–10 V) modes; the latter was ramped to at least double the sensory stimulation voltage. Then, 1 mL of ropivacaine hydrochloride (20 mg/10 mL) was injected through the cannula. The radiofrequency electrode was then reinserted into the cannula, and a lesion was made at a temperature of 80°C for 90 seconds using a radiofrequency generator (Electrothermal 20S Spine System, Smith & Nephew, London, GB). Selective denervation of specific facet joints was not performed; the L3/L4, L4/L5, and L5/S1 facet joints were denervated bilaterally in all participants.
(Left) Example of patient positioning, set-up of materials, imaging, and monitoring tools. (Right) Representative example of intrainterventional imaging: examples of x-ray-guided needle positioning.
Execution of Chemical Neurolysis With EA-95
The same setup was used for steroid infiltration and RFD. After positioning the 22G needle at the intersection of the superior articular and transverse processes of the target vertebrae, the upper outer quadrant of the pedicle, and the L5 dorsal ramus, the same needle was used to strike the junction of the superior medial sacral ala, just lateral to the superior articular process of S1, under fluoroscopy. Correct needle placement was ensured from the anteroposterior and lateral viewpoints. When the needle was in place, 0.5–1 mL of 2% ropivacaine was used to obtain a sufficient analgesic effect and to ensure that the needle tip was not positioned near the ventral ramus. Prior to the ethyl alcohol injection, each patient was asked about radicular pain or traction of the leg, which would have indicated incorrect needle placement, and the bevel opening was directed caudally to avoid the spread of the injectate into the intervertebral foramen. 1.5 mL of the EA-95 solution (B. Braun, Melsungen, Germany) was injected at each site. According to our guidelines, this was done in multiple steps and 0.5 mL of the solution was injected once over an interval of 30 seconds to avoid unwanted spread. In line with the literature, EA-95 was utilized.12,13 Prior to injection, the solution was stored under dark conditions at 4°C in accordance with the manufacturer’s recommendations. Again, we did not perform selective denervation of specific facet joints. In all patients, the L3/L4, L4/L5, and L5/S1 facet joints were denervated bilaterally.
Execution of Chemical Neurolysis with Gly-20
The procedure for Gly-20 neurolysis was the same as the one used for EA-95. Based on recommendations from the literature and our own experience, we used Gly-20 (glycerol anhydrous 3.0 g/15 mL, produced at our institution). Prior to injection, the Gly-20 solution was stored under dark conditions at 4°C.13
At our institution, a total of 1000 patients are treated annually using different types of x-ray–controlled semi-invasive infiltration therapies. All procedures are performed by experienced specialists with extensive experience in the field of pain management (>500 injections) and spinal surgery.
Outcome Parameters
The following outcome parameters were used based on information in the German spine database from the German Spine Society (DWG registry):
Patient characteristics: gender, age, date of presentation, date of intervention.
Pain characteristics: VAS, the Core Outcome Measures Index for the back (COMI-back), and the World Health Organization (WHO) classification of pain relief medication intake and opiate consumption. VAS-pain intensity was measured using the 11-point Numeric Rating Scale, where 0 indicates no pain and 10 indicates the worst level of pain. The COMI-back is a very brief instrument for assessing the main outcomes of importance in patients with back problems (pain, function, symptom-specific well-being, quality of life, and disability).14 COMI-back has subsequently been validated in many languages and is the outcome instrument of choice for back patients in the EUROSPINE international spine registry, “Spine Tango.”15
Additional outcome data: complications, need for subsequent operative interventions, and rehospitalization.
Statistical Methods
Statistical analysis was performed using SPSS 22.0 for Windows (Chicago, IL, USA). The differences between groups were calculated using χ2 or Fisher exact test for the ordinal data and t tests or the Mann-Whitney U test for continuous data; P values <0.05 were considered statistically significant.
Results
Patient Inclusion and Baseline Data
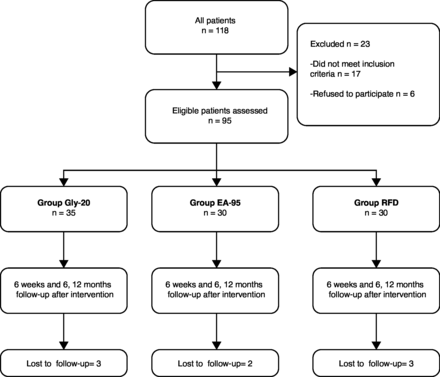
A total of 118 patients underwent lumbar facet denervation during the inclusion period; 23 were not enrolled because they met one or more of the exclusion criteria. Eventually, 95 patients were found to be eligible for the study. Of those 95 enrolled individuals, 35 were treated with glycerol injection therapy, 30 opted for RFD, and 30 preferred ethyl alcohol injection therapy. Loss to follow-up occurred in 8 patients during the 12-month observation period. Information on patient inclusion and grouping is illustrated in the flowchart presented in Figure 2.
Flowchart shows overview of patient enrollment, grouping, and follow-up. EA-95, ethyl alcohol 95%; Gly-20, glycerol 20%; RFD, radiofrequency denervation.
Prior to the intervention, no differences in the baseline parameters were seen among the 3 groups. Mean age was 63.7 ± 13.4 years. The patient cohort was predominantly female (60 female patients vs 35 male patients). Regarding pain medication, the WHO classification levels were similar among the groups, with an average score of 1.39 ± 0.55, and an overall VAS of 8.01 ± 1.58 was found. No differences in COMI-back scores among the study groups were observed. The baseline characteristics are shown in Table 1.
Patient characteristics and baseline parameters by group.
Short-Term Outcome Parameters
The documented pain medication levels according to the WHO classification system decreased in the patients in the Gly-20 and RFD groups, whereas increased pain medication requirements were seen 6 weeks after intervention in the EA-95 group. Six weeks after intervention, VAS was lower in all 3 groups and an overall postinterventional VAS of 5.36 ± 3.80 was found. Moreover, VAS after 6 weeks was significantly lower in the RFD group than in the EA-95 group (4.33 ± 2.83 vs 6.15 ± 3.21, respectively, P <0.05). Additionally, a trend was observed when comparing the Gly-20 injection with RFD; the mean VAS after 6 weeks was lower in the RFD group than in the Gly-20 group (4.33 ± 2.83 vs 5.67 ± 3.03, respectively, P = 0.08), but statistical significance was not reached. After 6 weeks, COMI-back did not differ significantly between the study groups.
After 6 months of observation, both VAS and COMI-back were significantly lower for the patients who underwent RFD than for those who underwent Gly-20 injection therapy (VAS: 4.42 ± 3.18 vs 6.64 ± 2.44, P <0.01 and COMI-back: 5.08 ± 3.45 vs 7.43 ± 2.26, P <0.01). The short-term outcome parameters are summarized in Table 2.
Short-term outcomes by treatment group.
Medium-Term Outcome Parameters
After 12 months of observation, the overall WHO pain medication consumption levels were found to have increased in comparison with the prior postinterventional time points, and the mean VAS also increased.
When comparing the differences among the 3 groups, VAS was significantly lower in patients treated with RFD than in patients treated with Gly-20 (4.72 ± 3.25 vs 6.68 ± 2.29). Furthermore, COMI-back was lowest in the patients treated with RFD compared with the other 2 therapies; however, statistical significance was not determined. Medium-term outcomes are shown in Table 3.
Long-term outcome in different treatment groups after 12 months.
Complications
In our study, no major complications were reported. All complications were short term and reversible. Most of the complicated courses were due to acute progression of pain symptoms upon intervention, requiring adaptation of analgesia. More specifically, 8 patients had to be hospitalized within 48 hours after the intervention due to acute pain progression, and they were treated using emergency infiltration therapy. Minor complications occurred most frequently in patients treated with the EA-95 injection (30% of patients in the EA-95 group vs 6.7% of patients in the RFD group and 2.9% of patients in the Gly-20 group). Eventually, according to our guidelines and due to suboptimal pain relief, 4 patients were referred to multimodal pain management (including psychological therapy), and 3 underwent endoscopic rhizotomy under general anesthesia.
Discussion
The key results of this prospective follow-up study are summarized as follows:
In patients with noninvasive therapy-resistant chronic lower back pain due to lumbar facet arthropathy, RFD therapy is associated with improved pain relief and quality of life compared with EA-95 or Gly-20 injections.
Application of chemical neurolysis with EA-95 was associated with an increased occurrence of minor complications compared to treatment with RFD therapy or Gly-20 injections.
In the absence of contraindications (such as a pacemaker or cochlear implants), RFD should be considered the treatment of choice in patients with chronic low back pain. If chemical neurolysis is indicated, we believe that Gly-20 injections should be the preferred treatment option given the large number of documented complications in patients treated with EA-95.
The current study focused on both RFD and chemical alternatives. RFD therapy aims to dampen nervous system-related noxious transmission, and it is considered an established, minimally invasive treatment method for chronic low back pain. Its efficacy has been demonstrated in several clinical trials.16,17 In short, RFD entails an electricity-generating source that transfers energy to an insulated electrode that contacts the tissue. The average generated temperature varies between 60°C and 80°C. The lesion size depends on the size and diameter of the needle tip, along with the temperature generated. The exposure time does not alter the tissue lesion size. Temperature-controlled radiofrequency is the preferred mode because it produces more standardized lesion sizes in comparison with voltage-controlled settings. The potential risks of this treatment are primarily related to needle insertion: local bleeding, local infection, and collateral damage to local structures. More specific to radiofrequency, there have been reports of transient burning pain or numbness and muscle weakness. Skin burns are a risk if the equipment is misused or damaged.18 Postdenervation neuritis has also been reported in the literature and is described as a sunburn-like feeling that usually resolves weeks after the procedure.13 Overall, the complication rates of RFD are generally low, ranging from 1% to 6.5%. No long-term complications were found in our literature review.19
In addition to RFD, the current study focused on chemical neurolysis, which is widely accepted in the field of oncological pain management. The benefits of chemical neurolysis are considered to largely outweigh its risks. However, according to current guidelines from the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine, chemical denervation should not be routinely used for the treatment of nononcological chronic pain.9,13 In spite of this, chemical neurolysis has been considered a feasible treatment option in patients with chronic lumbar facet joint pain in our level 1 spine center.
Chemical neurolysis is most often performed with phenol, ethyl alcohol, or, less commonly, glycerol. Hypertonic saline, ammonium salt solutions, and chlorocresol have also been used in the past.20 In short, these agents are believed to disrupt the transmission of pain signals. More specifically, phenol diffuses into the axon, where it causes Wallerian degeneration of the proteins. Phenol effects consist of a combination of neurotoxicity and ischemia. Histological analyses have demonstrated nonselective nerve destruction, muscle atrophy, and necrosis after undergoing phenol injections. In contrast, alcohol produces a nonselective destruction of nervous system tissue by precipitating cell membrane proteins and extracting lipid compounds, resulting in demyelination and, eventually, Wallerian degeneration. Alcohol neurolysis evokes an initial burning sensation along the nerve root, which is followed by numbness along the same distribution pattern. Because ethyl alcohol lacks local anesthetic properties, such as those seen in phenol injection fluids, it is usually more painful upon injection. However, the intensity and duration of nerve blocking are less pronounced with phenol than with alcohol. Phenol injections are not performed at our institution; we prefer to use Gly-20 solutions. Glycerol is a highly viscous neurolytic agent. It is an established blocking agent acting at the Gasserian ganglion and is frequently used to treat trigeminal pain. However, previous studies have suggested that its analgesic effects are temporary and reversible.21,22
Chemical neurolysis has some relevant disadvantages. Potential complications include cardiac rhythm disturbances, hypotension, skin and nontarget tissue necrosis, and central nervous system excitation. Furthermore, in the specific case of ethyl alcohol neurolysis, postneurolytic chemical neuritis with extensive burning pain in the distribution area of the nerve has been documented. Unfortunately, incidences of postneurolytic chemical neuritis are high, and rates of up to 10% have been reported. It has been hypothesized that this complication is due to incomplete destruction of the somatic nerves and subsequent painful regeneration of those nerves.13,22,23 The findings of the current study emphasize the relevance of this issue, as a significant proportion of patients treated with ethyl alcohol have suffered severe pain progression, some even requiring emergency hospitalization.
In accordance with our protocols, in the case of profound pain reduction upon intervention, we decided to reduce the preprocedural pain medication directly. Postprocedural escalation of pain medication was not performed routinely. It is tempting to speculate that to overcome transient pain escalation in individuals selected for chemical neurolysis, these patients would benefit from routine temporarily increased oral analgesics.
Another potential risk of chemical neurolytic agents is the uncontrolled spread of the injectate. Unwanted diffusion of fluids from the paravertebral gutter into adjacent areas (including the neuroforamina, the epidural space, and even the cerebrospinal fluid) may be harmful and is difficult to control. Cases of chemical neurolysis-related persistent paraplegia have been documented.24 In the current study, complications with motor paralysis or paraplegia associated with different modes of chemical neurolysis did not occur; however, several patients experienced transient dysesthesias and hyperesthesias after the procedure.
In our comparative study, RFD was associated with an overall favorable outcome in comparison to EA-95. This is in contrast with a cohort study performed by Joo et al.6 They demonstrated that alcohol ablation resulted in prolonged pain relief and improved quality of life compared with repeated RFD therapy in patients with recurrent thoracolumbar facet joint pain during a 24-month observation period. The discrepancy between their findings and our data is most likely due to differences in the patient inclusion criteria. More than 50% of the patients in the study conducted by Joo et al either underwent previous radiofrequency therapy or spinal surgery or had been diagnosed with severe kyphoscoliosis. Their patient cohort was very heterogenous, which was mentioned by the authors as a key shortcoming of their study. Consequently, their cohort differed significantly from the patients in our study, as all the previously mentioned interventions and diagnoses are absolute contraindications.6
Nevertheless, it is tempting to hypothesize that chemical neurolysis with ethyl alcohol is preferable in patients with major concurrent spinal diagnoses and/or previous surgical intervention, whereas RFD is more beneficial in patients with isolated chronic low back pain. Unfortunately, Joo et al used a Binary Outcome Scale to determine pain relief, so it is not possible to compare the overall effectiveness of the treatments used in both studies.6 The documented overall effectiveness of RFD 4 weeks after the intervention varied between 42% and 93%. Long-term effectiveness, defined as at least 12 months with pain relief of 50% or more, also varied between 47% and 87%.17,25,26 These outcomes are in line with our observations; after RFD , VAS dropped significantly from 8.00 ± 1.46 to 4.33 ± 2.83 and 4.72 ±3.25 after 6 weeks and 12 months, respectively.
Interestingly, pooled postinterventional WHO and VAS values were even worse than baseline values at specific timepoints. This is a direct result of the markedly inferior results of EA-95 therapy in this specific subgroup. As previously described, the cohort treated with EA-95 chemical neurolysis had clearly inferior results regarding pain medication requirements and VAS than other study conditions.
The current prospective study has some limitations. It provides only 1 year of follow-up data. However, because we defined strict inclusion and exclusion criteria, we were able to assemble a homogenous patient cohort. Moreover, as a result of meticulous data collection, the number of missing parameters is minimal. Randomization was not utilized; however, comparable study groups were constructed because the patients’ treatment preferences were diverse. Consequently, the baseline parameter criteria did not show any significant differences with respect to the final outcomes.
Conclusion
The current prospective follow-up study reveals that in patients with recurrent low back pain due to lumbar facet arthropathy, RFD therapy is associated with enhanced pain relief and quality of life compared with chemical neurolysis modalities. Furthermore, more complications were observed in the patients treated with EA-95 than the other 2 treatment options. In our view, in the absence of contraindications (such as a pacemaker or cochlear implants), RFD should be considered the treatment of choice in patients with chronic low back pain that is unresponsive to conservative treatment. If RFD is contraindicated, we suggest utilizing chemical neurolysis with Gly-20. Further research (including randomized trials) is necessary to confirm our findings and to identify specific patient groups who will benefit most from semi-invasive interventions for lumbar facet arthropathy that is resistant to noninvasive therapy.
Footnotes
Funding The authors report no funding related to this work.
Declaration of Conflicting Interests The authors report no conflicts of interest in this work.
Disclosure The authors report no financial disclosures related to this article.
Ethics approval The study was approved by the regional Ethics Committee (Aerztekammer Nordrhein, Germany) under file number 2016448.
- This manuscript is generously published free of charge by ISASS, the International Society for the Advancement of Spine Surgery. Copyright © 2022 ISASS. To see more or order reprints or permissions, see http://ijssurgery.com.