cytopreparation techniques part 2
- 1. Duhok University Faculty of medical science School of health science Dr. Azad Mustafa Ahmed Lecturer, FIBMS (Path) University of Duhok, faculty of medical science Cytology lecture series
- 3. PAPANICOLAOU (PAP) STAINING METHODS •The polychrome Papanicolaou (Pap) staining method has gained worldwide acceptance for cytologic samples. Georgios Nikolaou Papanikolaou May 13, 1883 – February 19, 1962
- 4. Principles •Stains include: –Nuclear staining: Hematoxylin –Two cytoplasmic counter staining: •Orange G (OG)-6, OG-5 and OG-8 is an acidic dye, stains keratin a bright, intense orange. •Eosin Azure (EA), EA-36, AE-50 and EA-65 including three stains –Eosin Y –Light Green –Bismarck brown Y
- 5. Principles •Hydration and dehydration: –Hydration prepares the cell sample for uptake of the nuclear dye; –dehydration prepares the cell sample for uptake of the counterstains. •Dehydration and clearing solutions result in cellular transparency and prepare the cell sample for the final steps
- 6. Factors affecting staining quality of Pap stain: 1.Fixation 2.Types of staining solution and method used. 3.Time, expire date and PH of solutions 4.Quality of dehydrating agents 5.Number of slides used.
- 8. Stain Quality Control 1.A control slide, buccal smear or ThinPrep Papanicolaou, should be run as the first slide through the stain procedure (manual or automated). The slide is evaluated and adjustments are made to the staining times if needed, to achieve the best staining quality.
- 9. Stain Quality Control 2.All the alcohols should be checked daily for stain saturation and the alcohol closest to the stain discarded, the other alcohols moved up and a new replacement alcohol put in the last position. This should be logged on a daily maintenance schedule.
- 10. Stain Quality Control 3. Check for Hematoxylin Exhaustion: –As the stain is filtered, a deep purple ring should form at the top of the filter paper. If the ring is brown or brownish green, discard and use fresh stain. –In a jar of running water, place 5–6 drops of hematoxylin, if the water remains a deep purple, the stain is good. If the water changes color to brown or brownish green, the stain is oxidized and should be replaced.
- 11. RAPID STAINING PROCEDURES FOR FINE- NEEDLE ASPIRATIONS
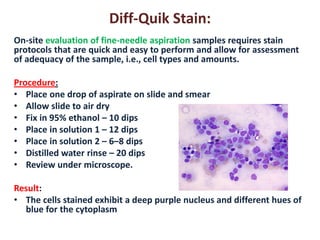
- 12. Diff-Quik Stain: On-site evaluation of fine-needle aspiration samples requires stain protocols that are quick and easy to perform and allow for assessment of adequacy of the sample, i.e., cell types and amounts. Procedure: •Place one drop of aspirate on slide and smear •Allow slide to air dry •Fix in 95% ethanol – 10 dips •Place in solution 1 – 12 dips •Place in solution 2 – 6–8 dips •Distilled water rinse – 20 dips •Review under microscope. Result: •The cells stained exhibit a deep purple nucleus and different hues of blue for the cytoplasm
- 14. ULTRAFAST PAPANICOLAOU STAIN This procedure was created to be as fast as the Diff-Quik stain, while producing results equivalent to or better than a routine preparation stained with the Papanicolaou stain. Procedure: •The specimen is taken, smeared on a slide, and allowed to air dry. •The slide is then placed in normal saline, •fixed in a mixture of 4% formaldehyde and 65% ethanol, •finally stained with Richard Allan Hematoxylin 2 and Cytostain. The staining sequence takes 90 s to complete.
- 16. CROSS-CONTAMINATION METHOD TO AVOID FLOATERS Floaters: contamination from one specimen to another •Occurs when a prepared sample has an overabundance of cells layered upon one another. The cells shed from the slide into the staining solution and can be picked up on other slides. •This will cause great distress among cytotechnologists and pathologists because of the probability of overcalling and misdiagnosing a patient.
- 18. The prevention of floaters can be reduced or eliminated by 1.Using charged slides when preparing specimens 2.Making monolayer preparations 3.Staining all preparations with a high degree of floaters separately 4.Filtering the stains daily 5.Staining cerebrospinal fluid (CSF) separately.
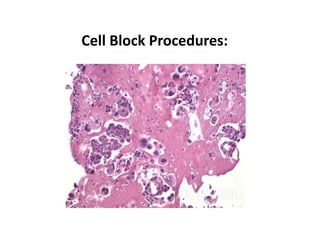
- 20. Cell blocks preparation •Cell blocks may be prepared from any sediment after centrifugation by various methods. • It is a procedure to convert cell sediment in to paraffin block in which further pathological procedures can be performed like immunohistochemistry (IHC).
- 21. Cell blocks preparation Procedure 1.Spin down specimen to achieve a concentrated cell button at 2400 rpm 2.Pour off supernatant 3.Add 5 cc of buffered formalin 4.Spin to fix specimen at 2400 rpm. Let specimen sit in formalin for 30-60 min 5.remove hardened cell button & place on blue histology tissue paper moistened with formalin 6.Fold the filter paper to secure the specimen & put inside a labeled cassette 7.submitte to histology laboratory.
- 22. Imprint Cytology Smears •It is a method for preparation of cytological smears from the fresh tissue. •It is used for rapid diagnosis like the frozen section, during surgery for intraoperative consultation.
- 23. Imprint Cytology Smears •Soon after an excision biopsy, the specimen is cut using a sharp scalpel blade. •Then take imprint smears by touching the cut surface with a clean microslide and fix immediately and rapidly stained.
- 24. Imprint cytology
- 25. `