Emulsifiers
- 1. Abd Karim Alias, 2013 EMULSIFIERS! Prof. Abd Karim Alias Universiti Sains Malaysia Photo courtesy of B, K, & G on Flickr
- 2. Abd Karim Alias, 2013© EMULSIFIERS! } Emulsifiers are substances which reduce the surface tension at the interface of two normally immiscible phases, allowing them to mix and form an emulsion. } Emulsifiers belong to the general class of compounds called surface- active agents or surfactants.
- 3. Abd Karim Alias, 2013© EMULSIFIERS! Functions of Emulsifiers } To promote emulsion stability, stabilize aerated systems, and control agglomeration of fat globules; } To modify texture, shelf life, and rheological properties by complexing with starch and protein components; } To improve the texture of fat-based foods by controlling the polymorphism of fats
- 4. Abd Karim Alias, 2013© EMULSIFIERS! How does emulsifier work? } Emulsifiers reduce surface tension between the two immiscible phases due to their molecular structure. They have both a polar group with an affinity for water (hydrophilic), and a nonpolar group with an affinity for oil (lipohilic). Ì Lipophilic tails are composed of C16 (palmitic) or longer fatty acids. Ì Polar head groups may consists of anionic, cationic, amphoteric, or nonionic functional groups.
- 5. Abd Karim Alias, 2013© How does emulsifier work? (-‐-‐-‐cont’) } The presence of both regions on the emulsifier molecule allows them to orient themselves at the phase interface & lower the interfacial energy that leads to instability. } Emulsifiers stabilize emulsions by means of monomolecular interfacial films & also by formation of steric and/or electrical barriers that prevent coalescence of the dispersed droplets. Without emulsifier With emulsifier
- 6. Abd Karim Alias, 2013© How does emulsifier work? (-‐-‐-‐cont’) Without emulsifier With emulsifier
- 7. Abd Karim Alias, 2013© EMULSIFIERS! Structure of emulsifier molecule
- 8. Abd Karim Alias, 2013© EMULSIFIERS! W/O emulsion O/W emulsion
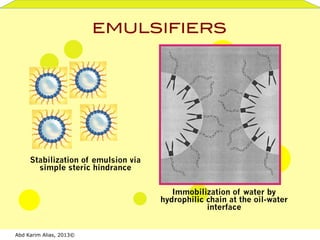
- 9. Abd Karim Alias, 2013© EMULSIFIERS! Immobilization of water by hydrophilic chain at the oil-water interface Stabilization of emulsion via simple steric hindrance