WESTGARD RULES
- 1. Dr. K. Selvakumar Medical Biochemist Mean +1SD +2SD +3SD -1SD - 2SD - 3SD
- 2. Definitions • Quality Control - QC refers to the measures that must be included during each assay run to verify that the test is working properly. – “The aim of quality control is simply to ensure that the results generated by the test are correct. • Quality Assurance - QA is defined as the overall program that ensures that the final results reported by the laboratory are correct. – However, quality assurance is concerned with much more: - that the right test is carried out on the right specimen, – and that the right result and right interpretation is delivered to the right person at the right time”
- 3. Definitions … • Quality Assessment - quality assessment (also known as proficiency testing) is a means to determine the quality of the results generated by the laboratory. Quality assessment is a challenge to the effectiveness of the QA and QC programs. • Quality Assessment may be external or internal, examples of external programs like EQAS.
- 4. Variables that affect the quality of results •The educational background & training of the scientific technologist •The condition of the specimens •The controls used in the test runs •Reagents •Equipment •The interpretation of the results •The transcription of results •The reporting of results
- 5. Errors in measurement • True value - this is an ideal concept which cannot be achieved. • Accepted true value - the value approximating the true value, the difference between the two values is negligible. • Error - the discrepancy between the result of a measurement and the true (or accepted true value).
- 6. Sources of error • Input data required - such as standards used, calibration values, and values of physical constants. – Expired, improper reconstitution, water used for reconstitution, pipetteing calibration error, improper storage, error in selecting test, machine, delay in run • Instruments used - accuracy, repeatability. • Observer imperfection - reading errors, blunders, equipment selection, analysis and computation errors. • Environment - any external influences affecting the measurement. • Theory assumed - validity of mathematical methods and approximations.
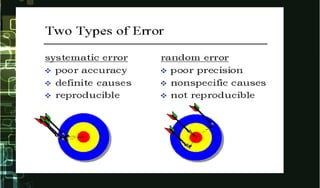
- 7. Random Error • An error which varies in an unpredictable manner, • Random errors create a characteristic spread of results for any test method and cannot be accounted for by applying corrections. • Random errors are difficult to eliminate but repetition reduces the influences of random errors. • Examples of random errors include errors in pipetting and changes in incubation period. Random errors can be minimized by training, supervision and adherence to standard operating procedures.
- 8. Random Errors x x x x x True x x x x Value x x x x x x x x x
- 9. Systematic Error • An error which, in the course of a number of measurements of the same value of a given quantity, remains constant when measurements are made under the same conditions, or varies according to a definite law when conditions change. • Systematic errors create a characteristic bias in the test results and can be accounted for by applying a correction. • Systematic errors may be induced by factors such as variations in incubation temperature, blockage of plate washer, change in the reagent batch or modifications in testing method.
- 10. Systematic Errors x x x x x x x x True x Value
- 12. • Accuracy is the closeness of a measured value to theAccuracy is the closeness of a measured value to the true value.true value. • For example, the measured density of water has becomeFor example, the measured density of water has become more accurate with improved experimental design,more accurate with improved experimental design, technique, and equipment.technique, and equipment. ACCURACYACCURACY Density of HDensity of H22O at 20° CO at 20° C (g/cm(g/cm33 )) 11 1.01.0 1.001.00 0.9980.998 0.99820.9982 0.998200.99820 0.9982030.998203
- 13. • Precision is the agreementPrecision is the agreement between repeated measurementsbetween repeated measurements of the same sample. Precision isof the same sample. Precision is usually expressed as a standardusually expressed as a standard deviation.deviation. PRECISIONPRECISION
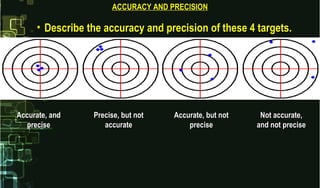
- 14. • Describe the accuracy and precision of these 4 targets.Describe the accuracy and precision of these 4 targets. ACCURACY AND PRECISIONACCURACY AND PRECISION Accurate, andAccurate, and preciseprecise Precise, but notPrecise, but not accurateaccurate Accurate, but notAccurate, but not preciseprecise Not accurate,Not accurate, and not preciseand not precise
- 16. • Given by Dr. James O. Westgard, of the University of WisconsinGiven by Dr. James O. Westgard, of the University of Wisconsin in an article in 1981 on laboratory quality control that set the basisin an article in 1981 on laboratory quality control that set the basis for evaluating analytical run quality for medical laboratories.for evaluating analytical run quality for medical laboratories. • Six basic rules in the Westgard scheme:Six basic rules in the Westgard scheme: • 1-3s, 2-2s, R-4s, 1-2s, 4-1s, and 10x.1-3s, 2-2s, R-4s, 1-2s, 4-1s, and 10x. • These rules are used individually or in combination (multi-rule) toThese rules are used individually or in combination (multi-rule) to evaluate the quality of analytical runs.evaluate the quality of analytical runs. • Detect Random error (imprecision or CV) & Systematic errorsDetect Random error (imprecision or CV) & Systematic errors (bias or inaccuracy, shifts & trends)(bias or inaccuracy, shifts & trends) Monitoring & Analysing Internal QCMonitoring & Analysing Internal QC Multi control QC rules (WESTGARD RULES)Multi control QC rules (WESTGARD RULES)
- 17. 112SD2SD or 1- 2s:or 1- 2s: ‘Warning’ Rule‘Warning’ Rule A single or any one of 2 or 3A single or any one of 2 or 3 control results falls outsidecontrol results falls outside ±2SD±2SD Either side - +/-Either side - +/- Applicable across runs & acrossApplicable across runs & across QC materialsQC materials Do not reject runDo not reject run Multi control QC rules (WESTGARD RULES)Multi control QC rules (WESTGARD RULES) ““Warning rule” - Alerts technologistWarning rule” - Alerts technologist to possible problems ‘to possible problems ‘ Start looking for other errors – lookStart looking for other errors – look at other control & previous dayat other control & previous day
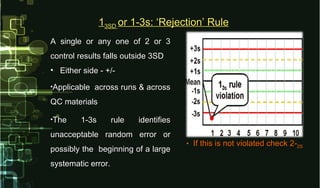
- 18. A single or any one of 2 or 3A single or any one of 2 or 3 control results falls outside 3SDcontrol results falls outside 3SD • Either side - +/-Either side - +/- •Applicable across runs & acrossApplicable across runs & across QC materialsQC materials •The 1-3s rule identifiesThe 1-3s rule identifies unacceptable random error orunacceptable random error or possibly the beginning of a largepossibly the beginning of a large systematic error.systematic error. • If this is not violated check 2-If this is not violated check 2-2S2S 113SD3SD or 1-3s: ‘Rejection’ Ruleor 1-3s: ‘Rejection’ Rule
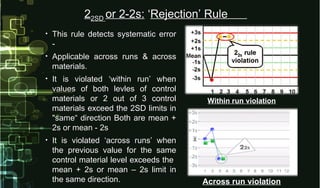
- 19. • This rule detects systematic errorThis rule detects systematic error -- • Applicable across runs & acrossApplicable across runs & across materials.materials. • It is violated ‘within run’ whenIt is violated ‘within run’ when values of both levles of controlvalues of both levles of control materials or 2 out of 3 controlmaterials or 2 out of 3 control materials exceed the 2SD limits inmaterials exceed the 2SD limits in "same“ direction Both are mean +"same“ direction Both are mean + 2s or mean - 2s2s or mean - 2s • It is violated ‘across runs’ whenIt is violated ‘across runs’ when the previous value for the samethe previous value for the same control material level exceeds thecontrol material level exceeds the mean + 2s or mean – 2s limit inmean + 2s or mean – 2s limit in the same direction.the same direction. Within run violation Across run violation 222SD2SD or 2-2s:or 2-2s: ‘‘Rejection’ RuleRejection’ Rule
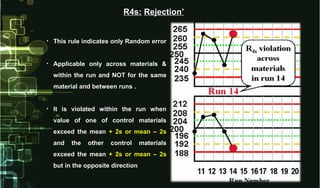
- 20. • This rule indicates only Random errorThis rule indicates only Random error • Applicable only across materials &Applicable only across materials & within the run and NOT for the samewithin the run and NOT for the same material and between runs .material and between runs . • It is violated within the run whenIt is violated within the run when value of one of control materialsvalue of one of control materials exceed the meanexceed the mean + 2s or mean – 2s+ 2s or mean – 2s and the other control materialsand the other control materials exceed the meanexceed the mean + 2s or mean – 2s+ 2s or mean – 2s but in the opposite directionbut in the opposite direction R4s:R4s: Rejection’Rejection’
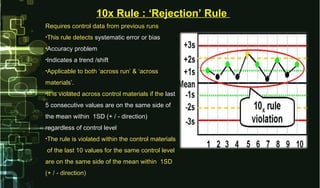
- 21. Requires control data from previous runsRequires control data from previous runs •This rule detectsThis rule detects systematic error or biassystematic error or bias •Accuracy problemAccuracy problem •Indicates a trend /shiftIndicates a trend /shift •Applicable to both ‘across run’ & ‘acrossApplicable to both ‘across run’ & ‘across materials’.materials’. •It is violated across control materials if theIt is violated across control materials if the lastlast 5 consecutive values are on the same side of5 consecutive values are on the same side of the mean within 1SD (+ / - direction)the mean within 1SD (+ / - direction) regardless of control levelregardless of control level •The rule is violated within the control materialsThe rule is violated within the control materials of the last 10 values for the same control levelof the last 10 values for the same control level are on the same side of the mean within 1SDare on the same side of the mean within 1SD (+ / - direction)(+ / - direction) 10x Rule : ‘Rejection’ Rule10x Rule : ‘Rejection’ Rule
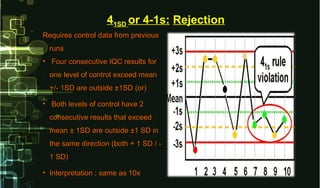
- 22. Requires control data from previousRequires control data from previous runsruns • Four consecutive IQC results forFour consecutive IQC results for one level of control exceed meanone level of control exceed mean +/- 1SD are outside ±1SD (or)+/- 1SD are outside ±1SD (or) • Both levels of control have 2Both levels of control have 2 consecutive results that exceedconsecutive results that exceed mean ± 1SD are outside ±1 SD inmean ± 1SD are outside ±1 SD in the same direction (both + 1 SD / -the same direction (both + 1 SD / - 1 SD)1 SD) • Interpretation ; same as 10xInterpretation ; same as 10x 41SD or 4-1s: Rejection
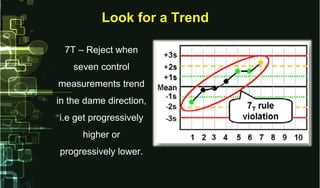
- 23. Look for a TrendLook for a Trend 7T – Reject when seven control measurements trend in the dame direction, i.e get progressively higher or progressively lower.
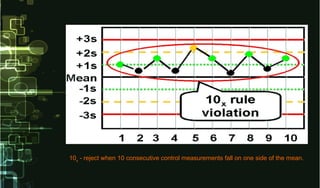
- 24. 10x - reject when 10 consecutive control measurements fall on one side of the mean.
- 25. Follow-up action There are three options as to the action to be taken in the event of a violation of a Westgard rule: • Accept the test run in its completeness - this usually applies when only a warning rule is violated. • Reject the whole test run - this applies only when a Rejection rule is violated.
- 26. When a rule is violated …When a rule is violated … Warning Rule – Use other rules to inspect the control points. Rejection Rule – Out of Control • Stop Testing • Identify & Correct Problem • Repeat Test on patient samples & controls • Don't report results until problem solved and controls indicate proper performance.
- 27. • Take action in a sequential manner to identify a problem – not in a randomTake action in a sequential manner to identify a problem – not in a random mannermanner • Analyse QC materialAnalyse QC material • Check if problem is resolved – If QC problem still persists take next stepCheck if problem is resolved – If QC problem still persists take next step • Record the problem, action takenRecord the problem, action taken • Technologists encouraged to perform corrective action steps byTechnologists encouraged to perform corrective action steps by themselvesthemselves RESOLVING QC PROBLEMSRESOLVING QC PROBLEMS
- 28. • 1. Check whether QC is out of control for many analytes using same1. Check whether QC is out of control for many analytes using same wavelengthwavelength • 2. Check whether the QC is out- of- control for both levels or any one level2. Check whether the QC is out- of- control for both levels or any one level for same analytefor same analyte • 3. Check whether reagent has reached its onboard stability period / nearing3. Check whether reagent has reached its onboard stability period / nearing itit • 4. Check whether QC material has reached its open vial stability period /4. Check whether QC material has reached its open vial stability period / nearing itnearing it • 5. Check whether latest calibration was OK5. Check whether latest calibration was OK STEPS IN RESOLVING QC PROBLEMSSTEPS IN RESOLVING QC PROBLEMS
- 29. • 1. Repeat Assay on control specimen using fresh aliquot of QC pool1. Repeat Assay on control specimen using fresh aliquot of QC pool • 2. Repeat assays on control specimen using a newly reconstituted set of control2. Repeat assays on control specimen using a newly reconstituted set of control • 3. Look for obvious problems – clots, blocks in probes, carryover reagent levels,3. Look for obvious problems – clots, blocks in probes, carryover reagent levels, mechanical fault,mechanical fault, • 4. Recalibrate instrument for the analyte in question reassay all controls4. Recalibrate instrument for the analyte in question reassay all controls • 5. Install a new lot of reagent bottle ( one or all),recalibrate & reassay all controls5. Install a new lot of reagent bottle ( one or all),recalibrate & reassay all controls • 6. Perform machine maintenance , recalibrate & reassay all controls6. Perform machine maintenance , recalibrate & reassay all controls STEPS IN RESOLVING QC PROBLEMSSTEPS IN RESOLVING QC PROBLEMS
- 31. • Out of control situations – set minimum criteriaOut of control situations – set minimum criteria • Outside ‘predetermined’ or ‘preset’ limits within a specified period – Use LabOutside ‘predetermined’ or ‘preset’ limits within a specified period – Use Lab established mean & rangeestablished mean & range • Set QC rules - ‘Multi QC rules’ or ‘Westgard Rules’Set QC rules - ‘Multi QC rules’ or ‘Westgard Rules’ • If precision is beyond acceptable limit - in terms of monthly & cumulative SD orIf precision is beyond acceptable limit - in terms of monthly & cumulative SD or CVCV • A pattern of inappropriate patient results with large number of abnormal valuesA pattern of inappropriate patient results with large number of abnormal values within a run / daywithin a run / day • A Result not matching with previous one (exceeding ‘delta check’ limits)A Result not matching with previous one (exceeding ‘delta check’ limits) MONITORING & DETECTING QC PROBLEMSMONITORING & DETECTING QC PROBLEMS MINIMUM CRITERIAMINIMUM CRITERIA